Russula cortinarioides Buyck, Adamčík, Lewis & V. Hofstetter
Etymology: referring to the gradually developing reddish brown colour of the gills, giving this species almost a Cortinarius-like aspect.
Holotypus: PC 0142175. Basidiocarps growing individually or in small groups. Cap 36–50(65) mm diam., regular, smooth, hardly pealing, glabrous, slightly depressed and not discolouring in the centre, unevenly tinged in colours that vary from grayish brown, yellowish brown to reddish brown, sometimes also predominantly dirty cream to whitish, particularly closer to the cap margin, greasy-viscose when wet, shiny even when dry, separable up to mid-radius, not striate at margin. Gills adnate to subdecurrent, brittle, unequal and irregularly alternating with lamellulae of one, two to sometimes three lengths, not widely spaced (8–15 l+L/cm at cap margin), 3–5 mm high, sometimes forked or anastomosing particularly close to the cap margin, whitish when very young, then rapidly cream to pale yellowish brown and finally turning vinaceous to deep brownish red when old; edges concolourous, even. Stipe central, shorter or more rarely longer than cap diam., 31–33(55)×7–11 mm, cylindrical, with the base often irregularly deformedwrinkled and sometimes narrowing (as in R. adusta), surface smooth, and although not pruinose, often more or less silveryglistening and whitish, rapidly dirty grayish when handled or rubbed gently as the initial whitish covering disappears and revealing a distinct longitudinal striation, relatively firm, spongy inside under a hard outer cortex which is grayish brown in section, not really chambered but irregularly hollowing in the central medulla. Context firm and fleshy in the cap center, only 2 mm thick at cap mid radius, whitish, turning brown with age, weakly reddening to becoming vinaceous when cut. Smell distinct and nauseous, somewhat alkaline (as in R. pseudolateriticola). Taste unpleasant and faintly astringent. Spore print white. Spores ellipsoid, (7.5–)7.8–8.1–8.4(–8.7)×(5.6–)5.8–6.1–6.4(–6.6) μm, Q=(1.25–)1.28–1.32–1.36(–1.4), with subreticulate to reticulate ornamentation of low, distinctly amyloid, obtuse warts 6–8 warts in a 3μm diam. circle on the spore surface], 0.2–0.3μm high, connected by fine line connections 2–5 lines in the circle] or fused in pairs or short chains [(1–)2–4 fusions in the circle],with a smooth, relatively large, nonamyloid suprahilar spot. Basidia (39–)46–50–57×8–9–10μm, 4-spored, narrowly clavate to subcylindrical; basidiola first cylindrical, then narrowly clavate. Subhymenium composed of densely septate, narrow cells. Lamellar trama with large sphaerocytes only present near the cap trama. Hymenial cystidia on gill sides abundant, [3000–4000 per mm2], (62–)67–80–93(–115)×7–8–9μm, also numerous but much shorter near the gill edge, measuring (26–)40–47–56×6–8(–10) μm, thin–walled, subulate, narrowly lageniform to subcylindrical, rarely clavate or lanceolate, sometimes mucronate with a short, 1–3(–5) μm long appendage, the interior partly filled with granular or slightly crystalline contents, turning reddish grey in sulfovanilin. Marginal cells not differentiated. Pileipellis 110–140μm deep, gelatinized, orthochromatic in Cresyl blue, ill-delimited from the underlying context. Incrustations absent. Hyphal extremities ascending, long and slender, becoming gradually denser downwards, uniformly cylindrical, sparsely branching, with obtuse terminal cells measuring (19–)30–41–60(–85)×(3–)4–5(–6) μm near cap margin, but narrower, 2.5–3.5–4(–4.5) μm diam. in the cap center. Pileocystidia numerous, usually very long (frequently>100μm) and originating deep in subpellis or cap trama where they continue as abundant cystidioid hyphae, one–celled, subcylindrical, apically attenuated and slightly mucronate, without or with one or two appendages (2–8μm long), often shorter, obtuse–rounded andmore clavate in cap center, thin–walled, (3.5–)4.5–5–6μm diam., filled with granular to yellowish-oily contents that do not react in sulfovanilin. Clamp connections absent in all parts.
Macrochemical reactions: stipe surface reacting strongly with guaiac, insensitive to FeSO4.
Specimens examined: UNITED STATES, Texas, Newton Co., Canyon Rim, near Mayflower, along State Highway 87, in Beech-Magnolia-Loblolly Pine woods, 28 July 2007, Buyck 07.133 (PC 0142175, holotypus); Ibid., 5 July 2002, Buyck 02.115 (PC0142176), 02.116bis (PC0142183), 29 Jul 2005, Buyck 05.084 (PC0124614), 28 July 2007, Buyck 07.131 (PC0142177 paratypus); Bleakwood, on Core‘s residence, in open Oak-Pine vegetation, N 30° 42.068′, W 93° 49.770′, Lewis 9185 (PC0142182). Montgomery Co., Conroe, in Oak-Pine woods, 27 July 2007, Buyck 07.103
(PC0124672), 07.104 (PC 0142178 paratypus), 07.105 (PC 0142179), 07.111 (PC 0142180 paratypus); Hardin Co., near Saratoga, Lance Rosier Unit, Big Thicket National Preserve, along Teel Cemetery road near cypress swamp among Cantharellus texensis in Oak-Loblolly Pine woods, N 30° 15.901′, W 94° 30.759′, ca 50 m alt., 16 June 2013, DP Lewis 10797 (PC 0142181), ibidem, 23 June 2014, Buyck, Hofstetter&Lewis leg., Buyck 14.024–14.027 (PC 0142213–PC 014226); Turkey Creek unit, Big Thicket National Preserve, along Kirby nature trail, N 30.47181-W 94.34888, 2 July 2014, Buyck 14.118 (PC 0142307)
Notes: The combined phylogenetic analysis of ITS, LSU & RPB2 sequences shows that the new species, Russula cortinarioides, is the most related to R. polyphylla Peck, the type species of subsect. Polyphyllinae Singer 1951 nom. inval. (Art. 39.1). This subsection was originally defined by the presence of numerous “gloeo-vessels or macrocystidioid oleiferous hyphae” in the upper layer of the cap cuticle, to distinguish them from other members of sect. Rigidae Fr. (= subgenus Heterophyllidia Romagn.). Singer (from 1951 onwards) recognized four species in this subsection: R. polyphylla (Peck 1898), R. magnifica (Peck 1903), R. polycystis (Singer 1939) and R. viridella (Peck 1906). Even before introducing this new subsection, Singer (1943) had already suggested the co-identity of the former two species, a synonymy he maintained up to the last edition of his Agaricales (Singer 1986), and in which he was followed by most mycologists. It is therefore difficult to understand why Singer placed Polyphyllinae in sect. Rigidae near subsect. Virescentinae (Schaeff.) Fr. because R. magnifica was described (Peck 1903) as a whitish species having unequal gills, with suggested affinities to a completely different group: subsect. Lactarioideae in subgen. Compacta Fr. (see Buyck and Adamčík 2013). Even Singer (as early as 1926) agreed with such a systematic placement for R. magnifica until he changed opinion in 1951 without any clear argumentation. The two other species, R. polycystis and R. viridella, have been placed in Virescentinae, until Singer (1951) transferred them to Polyphyllinae. R. polycystis was originally described as a new species of Virescentinae (Singer 1939) and this position has been maintained, although with some hesitation, when Bills (1984) reported on recollecting it for the first time since its original description. R. viridella has also been associated with Virescentinae (Singer 1932 and thereafter) not with standing its acrid taste. It is more than likely that both these species are unrelated to either of these subsections. It was Buyck (in Buyck et al. 2005) who, for the first time, pointed out the strong microscopical similarities between R. polyphylla and R. eccentrica Peck, a relationship that is fully supported (BS=100 %) by our phylogenetic analysis. The molecular data indicate that Polyphyllinae are neither related to Virescentinae, nor to Lactarioideae, but are closely related to subsect. Nigricantes Fr., a species group typically characterized by their, often first reddening, then blackening context. The closest, native American taxon to our new species seems to be R. densifolia var. paxilloides Peck, which differs from our species by the differentiation of marginal cells and in the less reticulate and less dense, slightly higher spore ornamentation (see Adamčík and Buyck 2014). Polyphyllinae differ from Nigricantes by the fact that their context is only reddening but never blackening, a feature most easily observed on the ageing hymenophore which turns pinkish to reddish brown at maturity. As morphology in our diagnosis, R. cortinarioides is most similar to R. eccentrica in the field but has denser gills, a feature that is even much more pronounced in the larger, nearly whitish R. polyphylla, its sister-species, which has crowded gills. In the field, R. cortinarioides is also very easily confused with R. compacta Frost because of the unequal gills, brownish colours, unpleasant smell, white spore print and overall similar habit. The latter differs from our new species in the hymenium bruising brownish upon handling but not slowly turning reddish to reddish brown as in R. cortinarioides; both species are also easily distinguished by the very different microscopic features of their pileipellis and our phylogeny distinctly shows both species to be quite unrelated. As a result of our molecular analysis, we therefore restrict Polyphyllinae in North America presently to R. polyphylla, R. eccentrica and the here newly described R. cortinarioides. Polyphyllinae is also known from Costa Rica (see Buyck and Halling 2004; Buyck et al. 2005) and the subsection is well represented in most other tropical and subtropical areas of the world (Buyck unpubl.). All species of this subsection are recognizable by their unequal gills becoming pinkish to reddish brown at maturity, a slowly, mostly weakly reddening, but not blackening context; they have gloeoplerous elements in all parts of the carpophore and possess very similar spores.
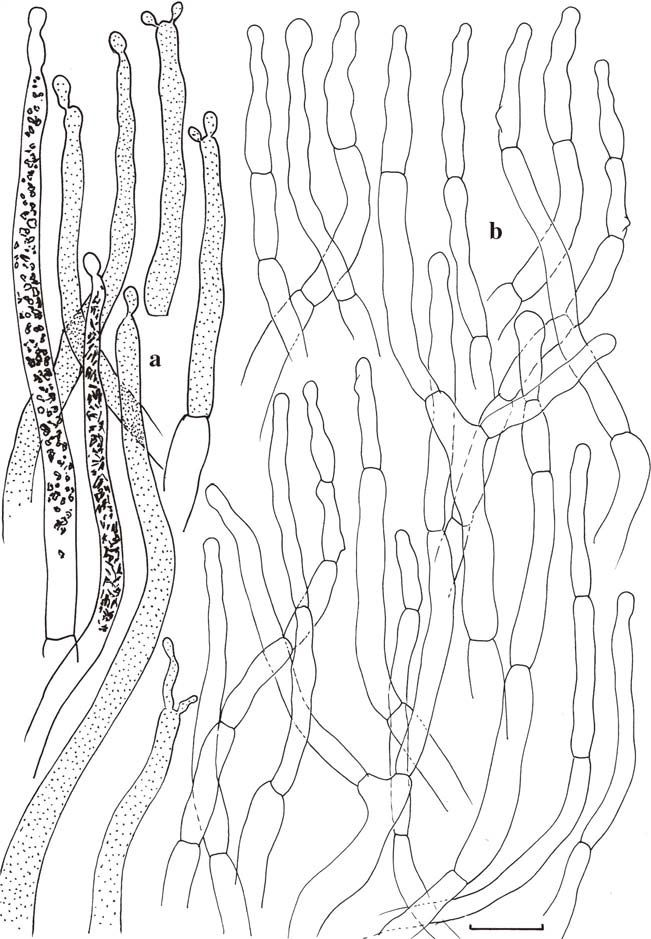
Russula cortinarioides (holotype). Microscopic features of the pileipellis. a Pileocystidia with indication of contents as observed in Congo Red for two elements, schematically in all other elements. b Hyphal extremities. Scale bars=10μm. Drawings B. Buyck
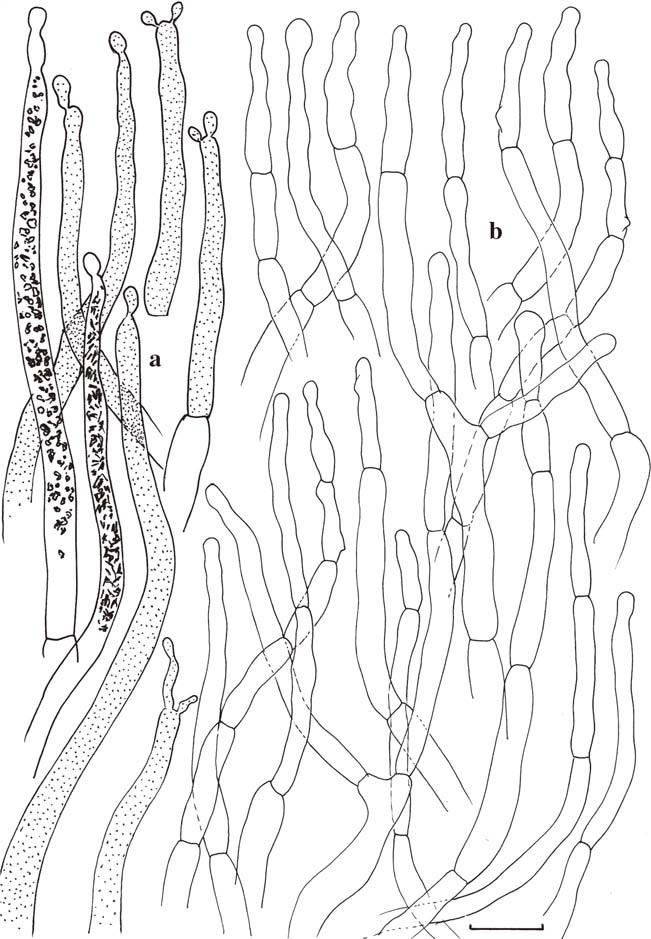
Microscopic features of the hymenium. a Basidia. b Basidiola. c Spores. d Gloeopleurocystidia. e Gloeocheilocystidia (no marginal cells differentiated). Gloeocystidia with indication of contents as observed in Congo Red for two elements, schematically in all other elements. Scale bars=10μm, but only 5μm for spores. Drawings B. Buyck
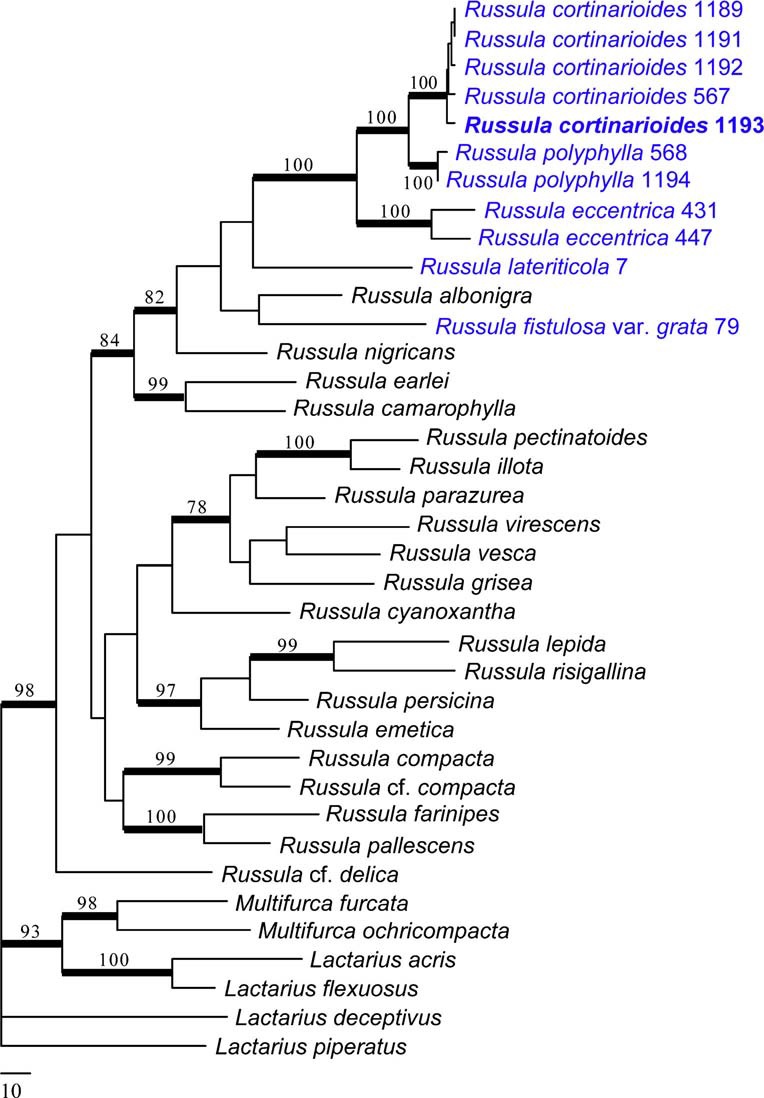
Phylogram generated from Maximum Parsimony analysis based on combined sequence data of RPB2, LSU, ITS for 31 Russula and six outgroup sequences (2 Multifurca spp., 2 Lactarius spp. and 2 Lactifluus spp.). Sequences used in this study have been sampled from a previous study (Buyck et al 2008) or newly generated for R. cortinarioides, R. polyphylla, R. eccentrica, R. lateriticola and R. fistulosa var. grata [see GenBank accession numbers KP033498–KP033508 (RPB2), KP033487 KP033497 (nucLSU), KP033476–KP033486 (ITS). Branches indicated in bold received significant (≥70 %) bootstrap support

