Montagnula infernalis (Niessl) Berl., Icon. fung. (Abellini).2: 68 (1896)
Basionym: Leptosphaeria infernalis Niessl, Inst. Coimbra 31: 13 (1883).
Index Fungorum number: IF 180704; MycoBank number: MB 180704; Facesoffungi number: FoF 00049
Saprobic on dead leaves in terrestrial habitats. Sexual state: Ascomata 220–280 × 250–310 µm (x̅ = 250 × 280 µm, n = 5), immersed to erumpent, gregarious or clustered, globose to subglobose, sometimes triangular in dried material, short ostiole always filled with hyaline closely adhering cells. Peridium 40–55 μm thick at sides, up to 80 μm thick near the apex, 3-layered, outer layer composed of heavily pigmented thick-walled small cells of textura angularis, apex thicker with smaller cells and thicker cell wall, thinner near the base; mid layer less pigmented, innermost layer of narrow compressed rows of cells, merging with pseudoparaphyses. Hamathecium of dense, 2–4.5 μm broad, narrow, septate cellular pseudoparaphyses. Asci 153–170 × 18–22 μm (x̅ = 157 × 20 µm, n=10), 8-spored, bitunicate, fissitunicate, cylindro-clavate to clavate, pedicel 28–60 μm long, with an ocular chamber best seen in immature ascus. Ascospores 24–29 × 9–11 μm (x̅ = 26 × 10 µm, n = 20), biseriate, oblong to narrowly oblong, straight or somewhat curved, reddish-brown to dark yellowish-brown, verruculose, with five transverse septa and one vertical septum in each middle cells, constricted at the primary and secondary primary septa. Asexual state: unknown.
Material examined – PORTUGAL, Coimbra lusitania, on leaves of Fourcroya longava (Agavoideae), February 1881, Moller (M 1183, holotype of Leptosphaeria infernalis).
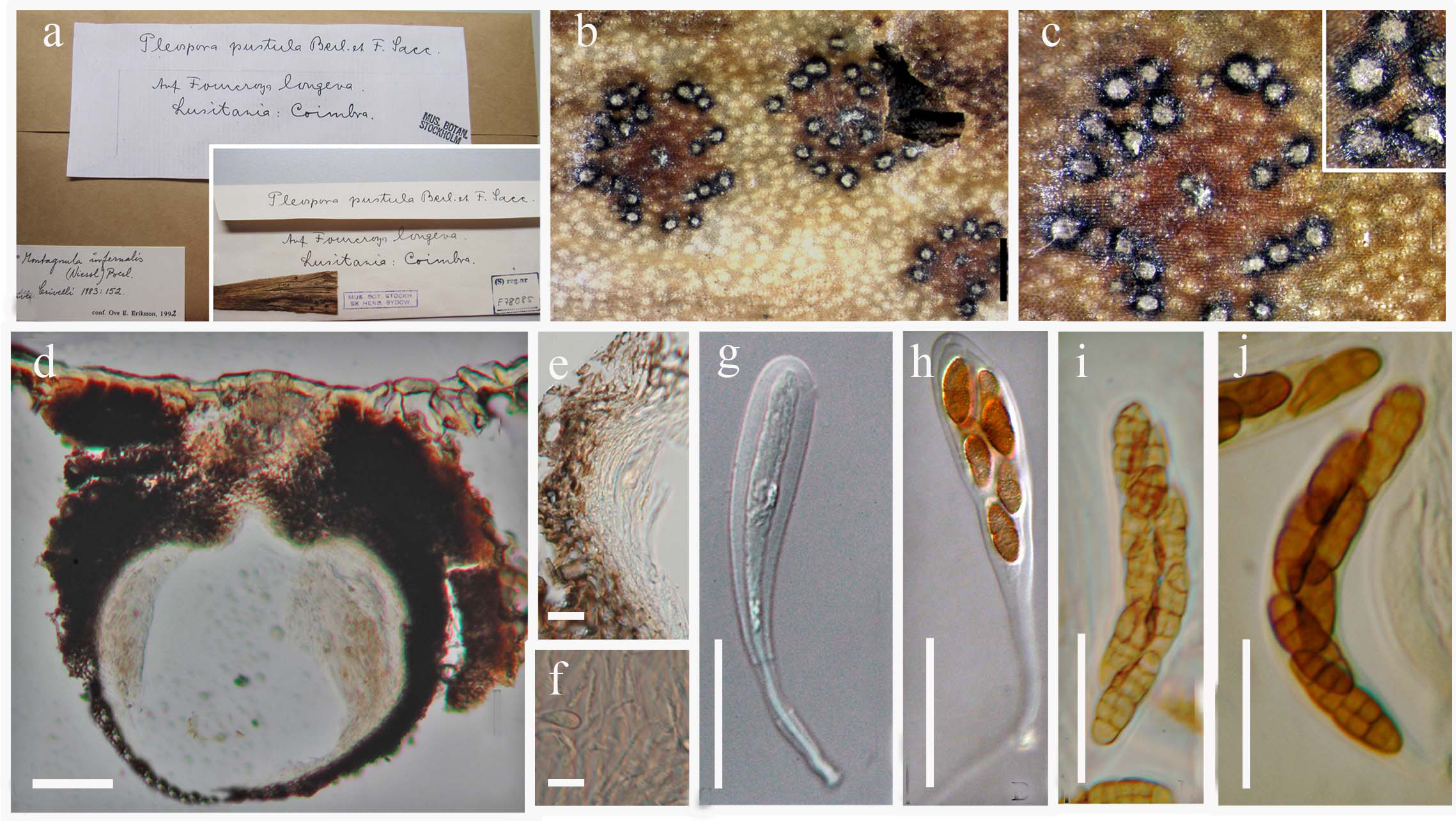
Fig. 12 Montagnula infernalis (M 1183, holotype of Leptosphaeria infernalis). a Fungus on the herbarium material. b–c Dry black ascomata on host surface. d Ascoma in horizontal section. e Layers of peridium. f Cellular pseudoparaphyses g immature ascus. h mature ascus with long pedicel. g–h Eight spored asci not visible. i–j Asci bearing muriform ascospores. Scale bars: a–c = 0.5 mm, d = 100 µm, e–f = 10 µm, g–j = 20 µm.
Notes – Montagnula was introduced by Berlese (1896) in order to separate two dictyosporous species, M. infernalis (Niessl) Berl., and M. gigantean (Mont.) Berl. from Pleospora, based on the presence of hyphal stromatic tissue over the ascomata and asci with a long pedicel (Barr 2001). Wehmeyer (1957) had placed Montagnula as a subgenus of Pleospora. Crivelli (1983) again treated Montagnula as a separate genus and divided the genus into two sub genera, i.e., Montagnula and Rubiginospora, whichare distinguished based on dark brown ascospores located on Agavaceae and reddish-brown ascospores on Poaceae, respectively (Barr 2001), but this proposal was not accepted by many mycologists. Subsequently, Leuchtmann (1984) and Aptroot (1995) included some phragmosporous and didymosporous species in the genus and eventually it became heterogenic (Zhang et al. 2012). Apart from the type species, some Montagnula species produce Aschersonia Mont. asexual morphs (Hyde et al. 2011). The genus presently has 28 epithets (Index Fungorum 2014). GenBank has 19 hits for the genus including putative strains of M. opulenta (De Not.) Aptroot (CBS 168.34), M. aloes Crous et al. (CPC 19671), M. rhodophaea (Bizz.) Leuchtm.(CBS 616.86), M. dura (Niessl) Crivelli (CBS 380.54), M. spartii (Fabre) Aptroot (CBS 183.58) and M. anthostomoides (Rehm) Leuchtm. (CBS 615.86).
One striking character of Montagnula infernalis is the very long ascal pedicel which develops once it is released from the ascomata. However, this character appears to have evolved more than once and can be found in Kirschsteiniothelia elaterascus Shearer which clusters with Helicascus (Shearer et al. 2009). The same character is also found in Xenolophium and Ostropella in the Platystomaceae (Mugambi and Huhndorf 2009). Montagnula opulenta is a didymosporous species, but phylogenetically closely related to those dictyosporous (Karstenula rhodostoma) and phragmosporous (Paraphaeosphaeria michotii) members of Montagnulaceae (Zhang et al. 2009). This might indicate that compared to other morphological characters, ascospore type is not a good character at the family level classification. Recent phylogeny based on multi-gene analysis has shown that the putative strain of M. opulenta forms a robust phylogenetic clade with species of Bimuria, Curreya, Didymocrea, Letendraea, Paraphaeosphaeria, Phaeodothis and Karstenula, which might represent a familial group (Schoch et al. 2006; Zhang et al. 2009, 2012).
Our phylogenetic data also shows that the putative strains of Montagnula rhodophaea (CBS 616.86) and M. spartii (CBS 183.58) clustered in Lentitheciaceae and Massarinaceae, respectively. The morphology and identification of these putative strains in GenBank as far as we can ascertain, cannot be checked, as they are not linked to any herbarium material, therefore we placed them in Lentitheciaceae incertae sedis and Massarinaceae incertae sedis, respectively. Montagnula dura (CBS 380.54) was excluded from our final analysis because in the preliminary analysis we observed that this strain clustered within Pleosporaceae (details not shown). Our phylogenetic results indicated that the putative strains of M. opulenta (CBS 168.34), M. aloes (CPC 19671) and M. anthostomoides (CBS 615.86) nested within Montagnulaceae and form a separate clade sister to the Kalmusia and Alloconiothyrium clades. The strain named Letendraea helminthicola (CHTAR43) resides in Montagnula. This is probably a misidentification of Montagnula sp. as Letendraea helminthicola. The morphology and identification of the putative strain (CHTAR43) of Letendraea helminthicola in GenBank as far as we can determine, cannot be checked, as they are not linked to any herbarium material thus preliminarily re-identified as Montagnula sp. Therefore based on morphology, coupled with available molecular data, we keep Montagnula as a distinct genus in Didymosphaeriaceae. Fresh collections of the M. infernalis and further molecular and morphological studies are desirable to confirm our results.