Leccinellum indoaurantiacum D. Chakr., K. Das, A. Baghela, S.K. Singh & Dentinger
Index Fungorum number: IF 551569 Facesoffungi number: FoF 02049
Etymology: Named after leccinoid specimens (collected from India) with an orange pileus like in Leccinum aurantiacum (Bull.) Gray.
Holotypus: D. Chakraborty & K. Das DC 14-019 (H).
Pileus 22–45 mm. diam.; hemisphaerical to convex; surface irregularly ridged and wavy, slightly glutinous in young fruit bodies, reddish orange (7B8) gradually paler (4A8) towards margin, orange to deep orange or light yellow to yellowish orange (5A7–8/ 4A5–6), turning deep orange to reddish orange (5–7A8) with KOH; margin entire with narrow sterile flap of tissue. Pore surface slightly depressed near stipe, pastel yellow (2A4) to lemon yellow, unchanging when bruised; pore 2–3/mm, rounded, compound. Tube 11 mm long, adnate-sinuate, light yellow (1A4), unchanging when bruised. Stipe 80–105×10–13 mm,central, often curved,with white basal mycelia, surface longitudinally striate-lacerate to squamulose or scabrate, with brownish yellow (5–6C8) squamules on yellowish background (2–3A4–5). Context solid in pileus and stipe; context (pileus and stipe) pale yellow (1A3), soon becoming distinctly pinkish white to light pink when exposed. Pileus context turning deep yellow (4A8) with KOH, reddish grey (12D2) with FeSO4 but, unchanging with guiacol. Stipe context turning reddish grey (12D2) with FeSO4, unchanging with KOH and guiacol. Odour and taste indistinct.
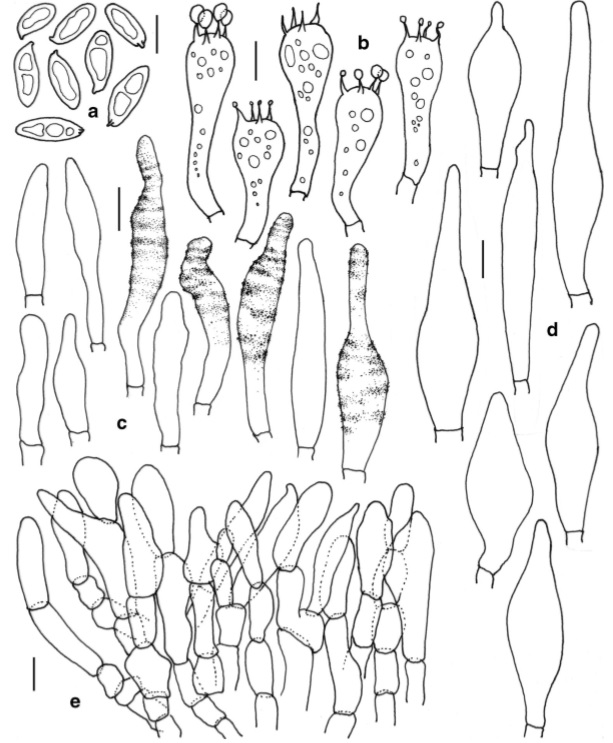
Basidiospores 14–19×5.8–7 μm (x =16.2×6.4, n=20, Q=2.19–2.52–2.92), oblong to subfusoid, inequilateral, smooth under light microscope, olive brown. Basidia 33– 53×11–16μm, 4-spored, clavate to subclavate; sterigmata 4–7×1–1.5μm. Hymenial cystidia 27–75×8.5–12μm, common, subcylindrical, subfusiform to subappendiculate, content insignificant, often encrusted, incrustations distinct, mainly located in concentric zones on neck. Tube edge fertile. Hymenophoral trama intermediate type. Pileipellis 110–150μm thick, ixotrichoderm, composed of erect septate hyphae, sometimes slightly interwoven; terminal cells 10–42×7–16μm, cylindrical to subfusoid to fusoid or ventricose, subclavate to clavate or rarely irregular, content slightly dense. Stipitipellis 100–130μm thick, fertile, composed of hyphae, basidia and cystidia; caulocystidia 47– 85×10–21μm, subfusoid, fusoid, ventricose, ventricoserostrate to appendiculate; caulobasidia similar to tube basidia but less in number. Clamp connections absent in all tissues.
Habitat and distribution: Under Betula sp. in Mema in chu and Kyangnosla areas, humid sub alpine mixed (broad leaf and coniferous) forests dominated by species of Abies, Betula and Acer. (Pseudotsuga, Tsuga, Abies). Producing basidiomata in the rainy season. Uncommon, Found in East district of Sikkim (India).
Material examined: INDIA, Sikkim, East district, Mema in chu area, 2 August 2014, D. Chakraborty & K. Das, DC14-019 (holotype,CAL; isotype, AMH); ibid., East district, Kyangnosla alpine sanctuary, 7 August 2014, D. Chakraborty & K. Das, DC14-030,(CAL);ibid., East district, Mema in chu area, 4 July 2015, D. Chakraborty, DC 15-007, (CAL).
Notes: LSU sequence data from the holotype (DC 14-019) was added to a dataset consisting of all LSU used in Wu et al. (2014). Multiple sequence alignment was achieved using the Practical Alignment using Sate and TrAnsitivity (PASTA) algorithm (Mirarab et al. 2014). The resulting alignment was used for maximum likelihood analysis implemented in RAxML v8.1.17 (Stamatakis 2006; Ott et al. 2007) using a GTRGAMMA model and branch support assessed using rapid bootstrapping set to terminate automatically based on the MRE criterion. The LSU sequence of DC14-019 was strongly supported (93 % bootstrap) in a clade with Leccinellum, Rossbeevera, Chamonixia, Octaviania, and Leccinum. The ITS sequence from the holotype (DC14019) was combined with sequences from related taxa downloaded from GenBank (Benson et al. 2013). Relevant GenBank sequences were downloaded following queries using search terms including the target taxon followed by BAND internal transcribed spacer, with model organisms excluded, including Octaviania (75 sequences), Chamonixia (21 sequences), Rossbeevera (92 sequences), and Leccinum (178sequences). After adding the sequence of DC14-019 and removing duplicate sequences, the final dataset consisted of 367 sequences. One sequence (AB848541) was on the complementary strand and was corrected before alignment. Multiple sequence alignment was achieved using the Practical Alignment using Sate and TrAnsitivity (PASTA) algorithm (Mirarab et al. 2014). The resulting alignment was used for maximum likelihood analysis implemented in RAxML v8.1.17 (Stamatakis 2006;Ottetal. 2007) using a GTRGAMMA model and branch support assessed using rapid bootstrapping set to terminate automatically based on the MRE criterion. The sequence of DC 14-019 was weakly supported (43 % bootstrap) with a clade composed of Leccinellum crocipodium, L. carpini, L. spp., and unnamed sequences. Although support was weak, the sequence clearly did not cluster with Leccinum s.s., and so we have provisionally included it within Leccinellum due to its putative phylogenetic affinities with other member of this genus. Leccinellum indoaurantiacum is characterized by yelloworange to orange-red typically hemisphaerical or convex pileus, yellow unchanging pore surface, striate squamulose to scabrate stipe with white basel mycelia, context quickly becoming pinkish white to light pink on exposure and presence of encrusted hymenial cystidia. In the field Boletus sinoaurantiacus M. Zang & R.H. Petersen appears to be similar with the present species but, the earlier grows on considerably lower altitudinal zone (1550–1680 m) and can be separated from the latter by showing unchanging context (pileus/ stipe) and absence of encrusted hymenial cystidia. Moreover, the association of B. sinoaurantiacus with the members of Fagaceae is quite distinct (Zang et al. 2001). Two other superficially similar species with an orange red pileus, Leccinum aurantiacum (Bull.) Gray (reported from North America) and L. insigne A.H. Sm., Thiers & Watling (reported from North America and also from India), may also create confusion with Leccinellum indoaurantiacum. However, the context of the Leccinum species are distinctly different, showing other colour reactions: context white initially becoming intermediate pinkish to wine-red then finally purple gray to blackish on exposure and pale blue with FeSO4 in L. aurantiacum; context white initially becoming purplish gray and then blackish without any intermediate reddening on exposure and bluish with FeSO4 in L. insigne (Bessette et al. 2010; Das and Chakraborty 2014). Moreover, L. aurantiacum has larger basidiomata (pileus 50–205 mm, stipe 100– 160×20 mm) and a pore surface that becomes brownish on bruising. Similarly, in L. insigne, basidiomata are more robust (pileus up to 15 cm diam., stipe 7–12×1–2 cm) with smaller (11–16×4–5μm) spores.
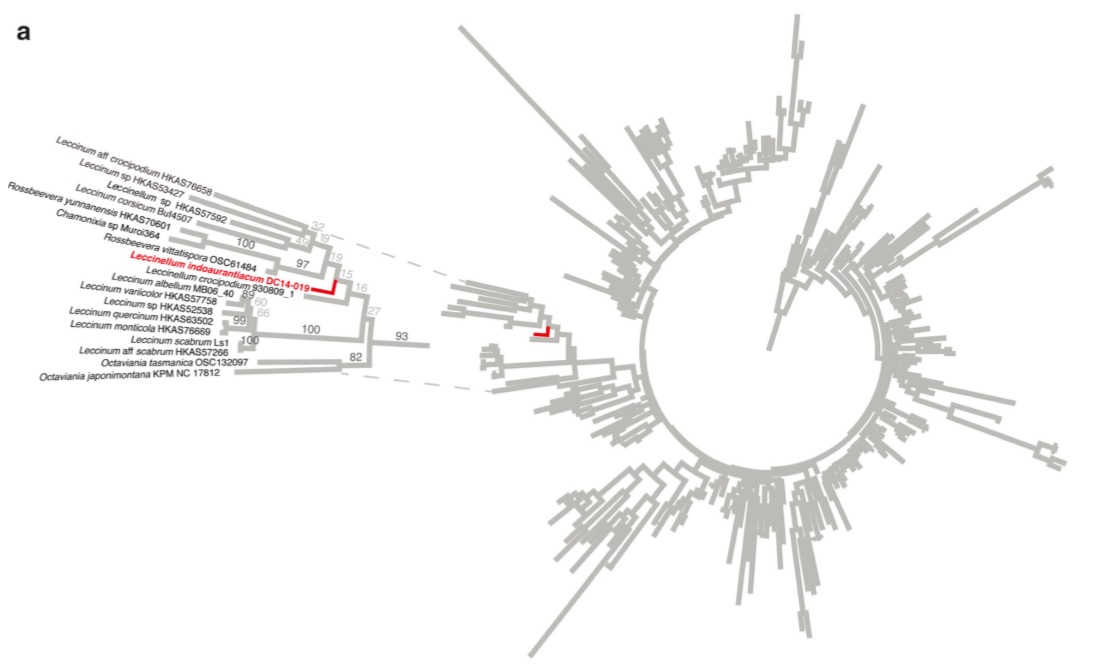
Phylogenetic placement of the new species Leccinellum indoaurantiacum a Best maximum likelihood circle phylogram recovered using RAxML of an LSU dataset including the new species Leccinellum indoaurantiacum (DC 14-019) and the alignment of Wu et al. (2014). Tree is rooted with Suillus spp. (HKAS57622 and HKAS57748) following the topology of Wu et al. (2014). The clade containing L. indoaurantiacum is magnified to the left. Numbers on branches are percent nonparametric bootstraps.
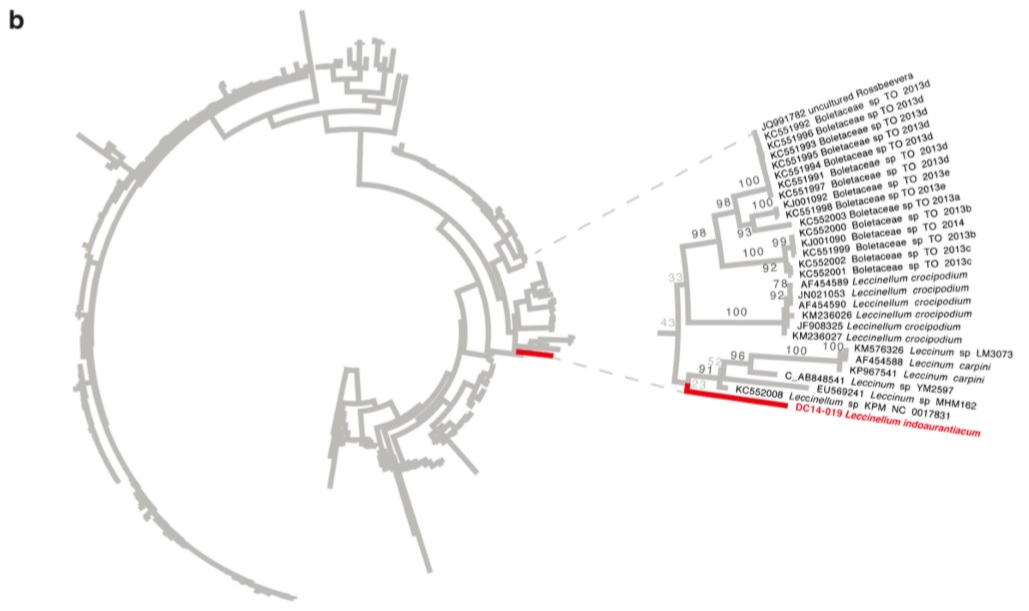
Phylogenetic placement of the new species Leccinellum indoaurantiacum b Best maximum likelihood circle phylogram recovered using RAxML of an ITS dataset including the new species Leccinellum indoaurantiacum (DC14-019) and related leccinoid taxa. Tree is rooted with Harrya chromapes following the topology of Wu et al. (2014). The clade containing L. indoaurantiacumis magnified to the right. Numbers on branches are percent nonparametric bootstraps.
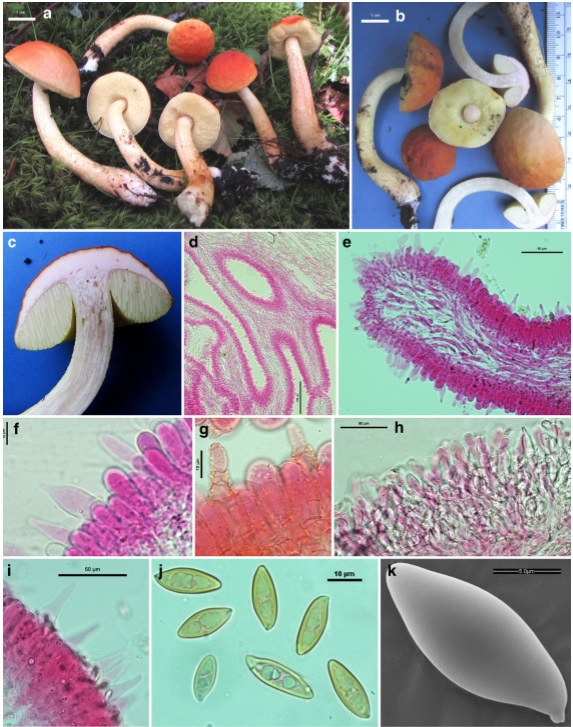
Leccinellum indoaurantiacum (holotype) a, b Fresh basidiomata c Pink context on exposure d Tube trama e Tube edge f Basidia g Hymenial cystidia h Transverse section through pileipellis i Caulocystidia j Basidiospores k SEM image of a basidiospore. Scale bars: a, b=1 cm, d=100μm, e, h, i=50μm, f, g, j=10μm,k=5μm