Anthostomella helicofissa Daranagama, Camporesi & K.D. Hyde, in Daranagama, Camporesi, Tian, Liu, Chamyuang, Stadler & Hyde, Fungal Diversity 73: 217 (2015)
Index Fungorum number: IF 809517; Facesoffungi number: FoF 00318
Etymology – In reference to the ascospore helical germ slit.
Holotype – MFLU 14-0235
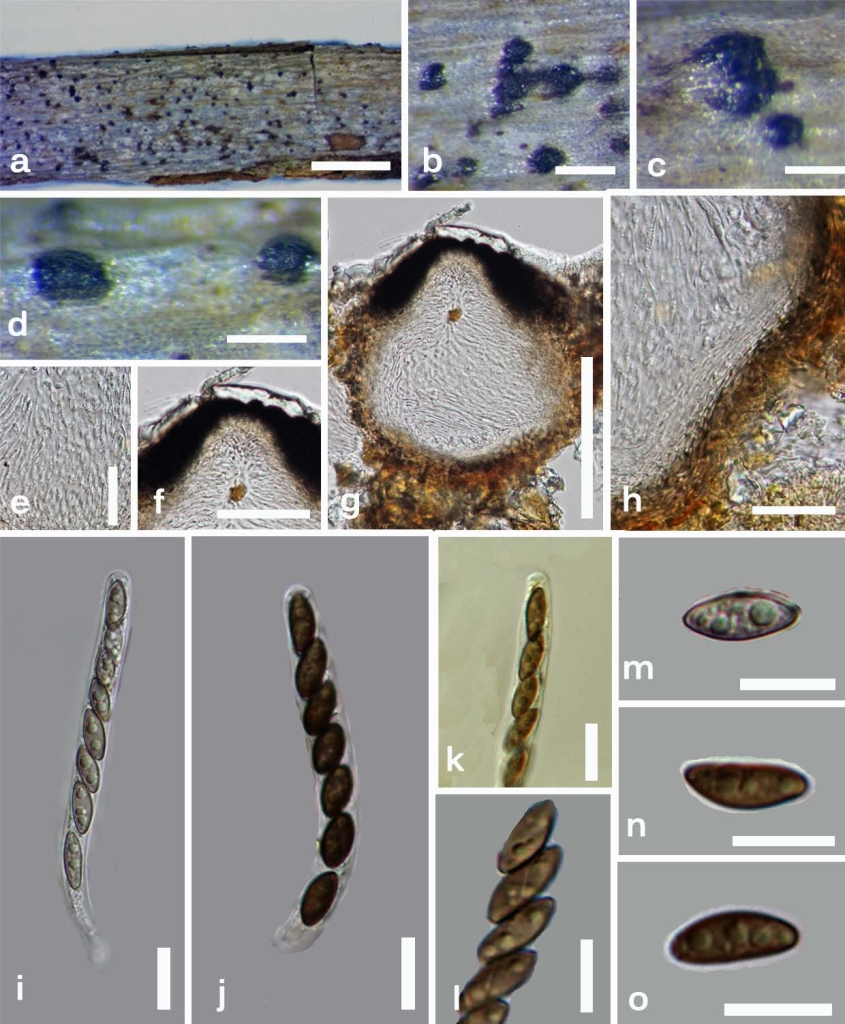
Saprobic on twigs. Sexual morph: Ascomata immersed, solitary or aggregated in small groups, visible as conical to pear-shaped areas, surrounded by host tissues and white remnants, in cross-section 150–195 × 170–200 µm (x̄ = 174 × 180 µm, n = 10), obpyriform, ostioles slightly papillate, ostiolar canal present, surrounded by carbonaceous tissues, 55–75 µm diam at base × 50 µm high (x̄ = 62 × 50 µm, n = 10). Peridium 35–40 µm diam. (x̄ = 37 µm, n = 10) with several cell layers, outwardly comprising thick-walled, dark brown cells of textura angularis and inwardly comprising thin-walled, hyaline cells of textura intricata. Paraphyses 3.5 µm diam. (x̄ = 3.5 µm, n = 30), filamentous, septate, numerous. Asci 110–185 × 11.5–14 µm (x̄ = 162 × 13 µm, n = 40), 8-spored, unitunicate, cylindrical, short-pedicellate, apically rounded, apical apparatus not observed. Ascospores 23–28 × 11.7–13 µm (x̄ = 25 × 12 µm, n = 40), uniseriate, equilaterally ellipsoidal, dark brown, unicellular, smooth-walled, thin gelatinous sheath present, less than 0.5 µm, germ slit, helical. Asexual morph: Conidiophores up to 50–80 × 1.3–2 µm diam. (x̄ = 70 × 1.5 µm, n = 20), simple, light brown, branched or single. Conidiogenous cells 3–5 × 0.5–1.6 µm high (x̄ = 4 × 1.0 µm, n = 20), few, small, disc-shaped, produced on denticles along conidiophores. Conidia 5–7 × 2–2.5 µm (x̄ = 6.5 × 2.1 µm, n = 20), hyaline, ellipsoidal, easily dehiscent, unicellular.
Culture characteristics – Colonies on Difco OA plates at 25 oC reaching the edge of 9 cm Petri-dish in 2 weeks, at first whitish grey, felty, azonate, with diffuse margins, developing black spots scattered all over the colony; reverse turning black or isabelline . Mycelia produce characteristic coil-like structures. Production of conidiophores dense, mycelia-producing conidiophores becomes yellow, stiff and puffy.
Material examined – ITALY, Province of Forlì-Cesena, Trivella-Predappio, on Cornus sanguineaba L. (Cornaceae), 5 January 2014, E. Camporesi IT1627 (MFLU 14-0235, holotype), ex–type cultures, MFLUCC 14-0173 = ICMP; Ibid, 5 January 2014, E. Camporesi IT1627 (PDD, isotype).
Notes – Anthostomella helicofissa shares certain characters with A. limitata Sacc., A. okatina Whitton et al., A. spiralis K.D. Hyde & B.S. Lu and A. umbrinella (De Not.) Sacc. Anthostomella limitata differs from the new taxon in having solitary, globose ascomata, with a central, periphysate, ostiolar canal, comprising brown fungal hyphae, a thin peridium, shorter asci with a J+, discoid apical apparatus and ellipsoidal ascospores with tapering ends. Anthostomella okatina has subglobose to broadly obclavate ascomata and the basal part of ascomata is often flat (Lu and Hyde 2000b). Anthostomella helicofissa has pear-shaped, thick-walled, obpyriform ascomata with a rounded base. Anthostomella spiralis has a comparatively thin peridium (5–10 µm) comprising brown, elongate cells, asci with a discoid, J+, apical apparatus and ascospores with a mucilaginous sheath, which is thick in the centre. Anthostomella umbrinella has larger, globose ascomata, consisting of dark intracellular fungal hyphae, asci with J+, apical apparatus and broadly ellipsoidal ascospores (Lu and Hyde 2000b). The asexual morph of A. helicofissa observed here is different from other Anthostomella species. The characteristic coil-like structures of the mycelium is another key feature of this new species.
Fig. 1 Anthostomella helicofissa (MFLU 14-0235, holotype). a Habitat on wood. b, c Ascomata in wood with white surrounding areas. d Ascomata in side view. e Paraphyses. f Ostiole. g Cross section of an ascomata. h Peridium. i, j Asci in water. k Asci inMelzer’s reagent lacking a J+ apical apparatus. l Ascospores with spiral germ slit. m–o Ascospores in water. Scale bars: a = 1 mm, b–d = 500 μm, e = 50 μm, f, g = 100 μm, h = 30 μm, i–o = 10 μm.