Rubroboletus demonensis Vasquez, Simonini, Svetasheva, Mikšík & Vizzini
= Boletus rhodopurpureus f. polypurpureus s. Ruiz Fernandez & Ruiz Pastor, Guía Micologica Tomo n. 4, Supl. Orden Boletales en España: 62 (2006)
= Boletus rubrosanguineus s. Calzada Dominguez 2007, Guía de los Boletos de España y Portugal: 163 (2007)
= Boletus legaliae s. Rodà, Funghi Aspromontani Comparati Boletales: 96 (right), 97 (top), countercover (2012)
Index Fungorum number: IF552526 Facesoffungi number: FoF 2953
Etymology: the specific epithet of the name of the taxon comes from the Latin word “demonensis” and refers to the ancient name “Valdemone”, which was one of the three valleys (“valli”) or real domains (“reali dominii”) in which the region Sicily was subdivided from the Muslim domination to the Bourbon period; the “Valdemone”, with respect to the “Val di Noto” and the “Val di Mazzara”, constitutes the north-eastern part of the Sicily, an area that closely corresponds to the actual habitat of R. demonensis. Furthermore, the epithet “demonensis” well reminds the peculiar features of the species, i.e. the flaming red colour of the cap and the pores, features shared with other close “devilish” species, belonging to the same genus (R. satanas). Moreover, unforeseeable circumstances would have wanted that one of the first growth stations of R. demonensis had been the locality “Pizzo Inferno” (i.e.: Hell Peak) on the Nebrodi chain, in the common of Floresta.
Holotype: MCVE 29081 (GS10244)
Pileus 60‒150 (‒240) mm broad, initially hemispherical, then convex, pulvinate, in the end almost applanate in mature basidiomes. Margin ± regular in young, soon uneven, ± undulate-lobate, sometimes distinctly lobate in mature specimens. Colour at the very beginning whitish, pale grey, pale ochre (4/A1–B2), soon, starting from the margin, dirty pale pink-lilac (5–12/A1–B2), then strongly variable from light grey (5–7/B1–C2), to pink-lilac (7–12/B2–C3), to purple-red (10–12/B3–C6, 11–12/C6–D8) depending on environmental conditions; with wet weather or in shady woods, the pileus shows particularly bright colours, tending to purple-blood red, quite uniformly distributed on the pileus surface (11–12/C8–D8). In some occurrences, red colour seems not to develop completely both in pores and stipe, and in meantime also the pileus colour stabilizes on a pink-lilac uniform colour (8–11/A3–B4), that becomes blood red when bruised. Conversely, in dry weather or in areas exposed to the sun, pileus colour is much more variable, with flesh pink colours sometimes fading to brownish-grey or to paler tone tending to cream-whitish (7–9/B2–D3). However, in the most of the basidiomes observed, large intensely reddish areas or wide blood red spots persist, even more intense following to scrubbing, touching or other manipulation. Surface typically tomentose and dry at the beginning, sometimes smooth and viscous in wet weather; the cuticle is not detachable from the underlying flesh. Often, in mature large basidiomes, the pileus appears pleasantly bright shining red, darker when bruised. Looking closely at the pileus surface, also with the aid of a lens, minute, in relief, concolorous scales can be noticed. This feature is shared with the close species R. rubrosanguineus, R. legaliae, R. pulcherrimus and R. sinicus. Stipe 80‒120 (‒150) × 40‒80 (‒90) mm, bulky, robust and wide, cylindrical, often gradually enlarged or clavate towards the base, sometimes also obese, not rooting. Surface in rare case yellow orange at the very beginning (4–6/A4–A6), with purplish base (9–10/A4–B6), then, passing through intermediate orange-red tones (7–8/A4–B6), in the end bright red, blood red, purple red, often darker at the base (9–10B7–D8, 9–11D6–D8), often with an evident deep yellow, or orange-yellow band (5–15 mm wide) in the upper part. In some xanthoid aspects stipe does not develop red tones in the upper half, remaining yellow or orange yellow, but the stipe base is anyway red, purple-red, blood red. Bitten areas often show the deep yellow flesh colour, giving a chromatic effect reminiscent of R. rhodoxanthus. A sharp net, with regular meshes, is well distributed over at least ¾ of the height, darker than the colour of the ground; the meshes are usually larger and the ribs usually thicker than those of B. rubrosanguineus or B. legaliae, giving to the net a general rougher appearance with respect to the ones of the close species. The extreme base of the stipe is covered with a whitish grainy, furfuraceous over layer, particularly evident in basidiomes collected on moist grounds. Tubes 5‒12 (‒20) mm, having average length, free at the stipe, from deep yellow to olive green, discolouring blue when cut. Pores small, round, usually from the beginning purple-red, dark red (10–11/C7–D8, 11–12/D7–F8), dull blue when touched, in aged basidiomes orange-yellow towards the margin of the pileus. In some collections, young specimens have yellow pores, that with age develop only an orange, red-orange colour, reminiscent that one of typical R. legaliae. Flesh firm, yellow, deep yellow, lemon yellow (3/A4–A8), particularly intensive in both stipe and pileus wounds; when exposed, quickly turning sky-blue, blue, deep blue (21–23/A4–B6), due to discolouring not particularly intensive, sometimes weak, then fading to a pale grey-cream colour. In some cases, in the exposed pileus or mostly in the lower part of the stipe some beetroot shades may appear. Sub-hymenophoral layer concolorous. Taste sweetish, slightly acidic. Smell weak, fungal and pleasant. Spore print tobacco-brown. Amyloid reaction in the flesh at the stipe base, according to Imler’s procedure (Imler 1950): the tissues at the stipe base are not amyloid in all the collections if examined microscopically. As in R. legaliae and R. rubrosanguineus, sometimes the amyloid reaction could be macroscopically “positive” (Cheype 1983) due to the absorption of the Melzer’s regent in the tissues. Basidiospores smooth, fusiform ellipsoid, with a more or less pronounced suprahilar depression, (12.5‒) 12.6‒13.6 (‒13.9) × (4.6‒) 4.7‒5.1 (‒5.1) µm (363/7/7), on average 13.1±0.5 × 4.9±0.2 µm, Q = (2.53‒) 2.57‒2.79 (‒2.89), Qm = 2.68±0.11, from light yellow to yellow-brownish in KOH. They may have different shape in different collections, despite the molecular investigation has confirmed them as belonging to the same species. In some collections, they are ellipsoid, more elongate with respect to those of R. legaliae, smaller both in width and in length with respect to those of R. rubrosanguineus but with almost the same Q ratio; in other collections, they are more fusiform, with the apex opposed to apiculum more tapered and acuminate. In some collections, lots of irregular spores, with various, irregular protuberances, may also occur. Basidia mostly 4-spored, hyaline, (23.8‒) 31.3‒47.7 (‒62.6) × (4.2‒) 7.7‒12.7 (‒13) µm (34/2/2). Facial cystidia fusiform, versiform, sometimes lageniform, hyaline, (15.5‒) 29.5‒44.7 (‒50.7) × (4.2‒) 5.3‒7.7 (‒9.5) (64/2/2). Marginal cystidia similar to facial cystidia but smaller, mostly versiform than fusiform or lageniform, hyaline (15.8‒) 20‒29 (37.2) × (3.5‒) 4.3‒6.3 (‒7.9) (46/2/2). Pileipellis an entangled trichodermium, tending to a cutis, often gelatinized in mature individuals, consisting of thin, cylindrical elements, collapsing gradually during development of the basidiomes. Terminal elements (33.3‒) 32.9‒42.9 (‒44.3) × (5‒) 5.2‒5.9 (‒6.9) µm (138/5/5), Q = (6.6‒) 6.4‒8.1, Qm = 7.3, with rounded or tapered tip, sometimes clavate, rarely capitulate or pear-shaped, with pale yellow vacuolar pigment and incrusted pigmented here and there, mostly in the deeper and closer to hypoderma hyphae. Hymenophoral trama boletoid in the sense of Singer (1965, 1967). Clamp connections absent.
Habitat: Rubroboletus demonensis is typical in acid and silicic soils, thermophilic, in summer. It grows in groups of small basidiomes in warm deciduous forests, in mountains or in some cases in mixed deciduous and coniferous woods (Pinus nigra and Taxus baccata), never with pure conifers. It prefers mesic forests of deciduous oaks (Quercus pubescens sensu lato, Q. cerris, Q. congesta and Q. virgiliana), rarely with holm (Q. ilex). It is common in chestnut woods (Castanea sativa), pure or mixed, and at higher elevations with beech (Fagus sylvatica). It is usually collected in June after the late spring rains and, not liking the summer drought, it disappears during the hottest period of July and August, then appears again in September. During the warmer autumns, it can be observed until the first half of October.
Distribution: R. demonensis was found in a rather delimited areal of the South Italy [(Sila Plateau (“Altopiano della Sila”) in Calabria] and Sicily, where it inhabits the mesomediterranean and supramediterranean zone of the mountain strip, characterized by a humid Mediterranean climate, with large rainfalls and Mediterranean humid forest well represented by deciduous forests of oak (Q. pubescens s.l.) and chestnut (C. sativa), some rare patches of evergreen forest with Q. ilex, with the presence of Pinus nigra ssp. calabrica, in an altitudinal range between 1200 m and 1600 m a.s.l.. Sicily seems to be the southern limit of distribution of the species. A unique collection from Greece (04.08.2014, near Nimea Village, Komotini, Rhodope Regional Unit, coord. 4112’42.2 N, 2531’16.5”E, alt. 650 m a.s.l., leg. D. Tragkos) with Q. petraea ssp. medwediewii, proved only by photographic material, represents the most eastern collection. The findings in Spain under Q. faginea and Q. ilex, until now attributed to Imperator rhodopurpureus var. polypurpureus (Ruiz Fernandez & Ruiz Pastor 2006) or R. rubrosanguineus (Calzada Dominguez 2007), seem likely referable to R. demonensis, and, if confirmed, might represent the western (Zamora, Salamanca) and northern (Navarra, Palencia, Huelva) limits of the distribution of the species. The main collection sites in Sicily are the north-eastern mountain range (Appennino Siciliano), the Nebrodi chain and the Madonie chains, but it has also been reported on the Etna volcano massif, where it grows even at higher altitudes. The most of the collections from Sicily comes from the commons of Cesarò, Floresta, Maniace and Troina in the Nebrodi Mountains Park, in the province of Messina.
Edibility: like other closely related species in this genus, it is probably poisonous if consumed raw.
Material examined: (GS refers to the personal herbarium of G. Simonini): Rubroboletus demonensis. ITALY – CALABRIA. 20 June 1999, Gambarie (RC), with C. sativa, coord. 3810’ N, 1550’ E, alt. 1300 m a.s.l., leg. C. Lavorato, GS2129. 9 June 2013, S. Domenica di Terreti (RC), with C. sativa, coord. 3810’21” N, 1547’48” E, alt. 900 m a.s.l., leg. P. Rodà, GS10708. SICILY. 26 September 2014, Portella Sella Maria (Nebrodi), Cesarò (ME), with F. sylvatica and Q. pubescens, coord. 3754’1” N, 1439’17” E, alt.1400 m a.s.l., leg. G. Vasquez, GS10400; 19 June 2015, Lago Biviere di Cesarò (Nebrodi), Cesarò (ME), with F. sylvatica, coord. 3757’6” N, 1442’50” E, alt. 1290 m a.s.l., leg. M.G. Pulvirenti, GS10242; 22 June 2015, Portella Sella Maria (Nebrodi), Cesarò (ME), with F. sylvatica and Q. pubescens, coord. 3753’57” N, 1439’15” E, alt. 1470 m a.s.l., leg. D. Milazzo, GS10243; 28 June 2015, Portella Sella Maria (Nebrodi), Cesarò (ME), with F. sylvatica and Q. pubescens, coord. 3754’1” N, 1439’17” E, alt. 1420 m a.s.l., leg. G. Vasquez, MCVE 29081, (GS10244, holotype); 20 August 2015, Piano della Cicogna, Cesarò (ME), with Q. cerris, coord. 3752’55” N, 1439’32” E, alt. 1350 m a.s.l., leg. M.G. Pulvirenti, GS10274; 20 September 2015, Piano della Cicogna, Cesarò (ME), with Q. cerris, coord. 3752’59” N, 1439’38” E, alt. 1320 m a.s.l., leg. M.G. Pulvirenti, GS10278.
Additional material examined (MM and JLC refer to the personal herbaria of M. Mikšík and J.L. Cheype): Rubroboletus legaliae. CZECH REPUBLIC – 17 July 2005, Lednice, chateau park, with Q. robur, 4848’44” N, 1648’25” E, alt. 230 m a.s.l., leg. M. Mikšík, MM 17705B2; 26 July 2008, Bechov-Svobodín, Mladá Boleslav, with Q. petraea and Carpinus betulus, 5026’7” N, 154’52” E, alt. 250 m a.s.l., leg. M. Mikšík, MM 26708B1; 6 August 2012, Dlouhopolsko, Nymburk, “Kněžičky” reserve, with Q. petraea, 509’40” N, 1520’24” E, alt. 240 a.s.l., leg. M. Mikšík, MM 6812B2; 9 August 2012, Dlouhopolsko, Nymburk, “Kněžičky” reserve, with Q. petraea, 509’46” N, 1519’47” E, alt. 250 a.s.l., leg. D. Kvasnička, MM 9812B3; 5 September 2015, Prodašice, Mladá Boleslav City, with Q. petraea and C. betulus, 5021’41” N, 157’44” E, alt. 250 m a.s.l., leg. J. Schneider, MM 5915B4. SLOVAK REPUBLIC – 8 July 2008, Hontianske Nemce, Krupina, “Štiavnické vrchy” natural reserve, with Q. pubescens, 4818’7” N, 1858’58” E, alt. 337 a.s.l., leg. M. Mikšík, MM 8708B1. HUNGARY – 27 July 1997, Pilisszentkereszt, Budapest, with Tilia sp., Q. cerris, Q. petraea, coord. 4742’ N, 1855’ E, alt. 450 m a.s.l., leg. A. Kiss, GS1881. ITALY – EMILIA-ROMAGNA. 16 September 1983, Pulpiano, Viano (RE), with Q. cerris, coord. 4430’32” N, 1033’31” E, alt. 514 m a.s.l., leg. unknown, GS12. SARDINIA. 31 July 1990, Case Floris, Desulo (NU), with Q. pubescens and C. sativa, coord. 4000’ N, 912’ E, alt. 1066 a.s.l., leg. G. Simonini, GS735; 9 November 1994, Cala Gonone, Dorgali (NU), with Q. ilex, coord. 4016’22” N, 936’47” E, alt. 187 m a.s.l., leg. A. Montecchi, GS1310. SICILY. 21 October 2013, Portella dei Bufali (Nebrodi), Cesarò (ME), with Q. cerris and Q. pubescens, coord. 3752’17” N, 1441’17” E, alt. 1180 m a.s.l., leg. G. Simonini, GS10112; 3 October 2014, Portella dei Bufali (Nebrodi), Cesarò (ME), with Q. pubescens and Q. cerris, coord. 3752’17” N, 1441’17” E, alt. 1180 m a.s.l., leg. G. Vasquez, GS10304; 28 June 2015, Portella Sella Maria (Nebrodi), Cesarò (ME), with F. sylvatica and Q. pubescens, coord. 3754’1” N, 1439’17” E, alt. 1420 m a.s.l., leg. G. Vasquez, GS10245. RUSSIA – 23 July 1983, vic. of township Soglasie, Penza Oblast, with Q. robur on the carbonaceous soil, leg. A.I. Ivanov, Le 200041; 8 August 2008, Voronka River Reservoir, vic. of Podgorodnie Datchi Village, Leninsky District, Tula Oblast, with Q. robur, coord. 54 6’5″ N, 3732’53” E, alt. 150 m a.s.l., leg. T. Svetasheva, GS10317; 14 July 2011 (also 16 August 2011, 27 July 2012, 5 August 2012), protected area “Nizovie Plushchan river”, “Galichia Gora” Reserve, Krasninsky District, Lipetsk Oblast, with Q. robur, leg. T. Svetasheva, L. Sarycheva; 1 September 2012, vic. of village Podgorodnie Datchi, Leninsky District, Tula Oblast, with Q. robur, coord. 54 6’5″ N, 3732’53” E, leg. T. Svetasheva, GS10311; 21 September 2012, vic. of khutor Leshchevsky, Leninsky District, Volga-Akthuba Floodplain, Volgograd Oblast, with Q. robur, coord. 4831’37.44″ N, 4451’42.90″ E, alt. 30 m a.s.l., leg. T. Svetasheva, GS10308; 14 October 2013, loc. Shutov Ugol, vic. of khutor Leshchevsky, Leninsky District, Volga–Akthuba Floodplain, Volgograd Oblast, with Q. robur, coord. 4831’50.29″ N, 4455’25.94″ E, alt. 20 m a.s.l., leg. T. Svetasheva, GS10304. Rubroboletus rubrosanguineus. AUSTRIA – 10 August 1998, Thurntaler, Sillian, with Picea abies, coord. 4645’54” N, 1224’39” E, alt. 1800 m a.s.l., leg. G. Simonini, GS1930. CZECH REPUBLIC – 17 August 2014, Francova Lhota, Beskydy Mountains protected area, with P. abies and Abies alba, alt. 650 m a.s.l., leg. J. Polcak, GS10285. FRANCE – date unknown, loc. unknown, with P. abies, leg. J.L. Cheype, HOLOTYPE JLC 83209. ITALY – EMILIA-ROMAGNA 20 July 1987, Le Borelle, Villa Minozzo (RE), with F. sylvatica, coord. 4418’ N, 1024’ E, alt. 1300 m a.s.l., leg. L. Cocchi, GS405; 31 July 1987, Le Borelle, Villa Minozzo (RE), with F. sylvatica, coord. 4418’ N, 1024’ E, alt. 1300 m a.s.l., leg. L. Cocchi, GS410; 1 September 1991, Costa delle Veline, Villa Minozzo (RE), with F. sylvatica, coord. 4416’ N, 1025’ E, alt. 1700 m a.s.l., leg. A. Montecchi, GS828; 8 August 1995, M. Prampa, Villa Minozzo (RE), with F. sylvatica, coord. 4420’ N, 1026’ E, alt. 1300 m a.s.l., leg. L. Cocchi, GS1387. TRENTINO–ALTO ADIGE 13 August 1997, Pochi, Salorno (BZ), with P. abies and F. sylvatica, coord. 4614’ N, 1115’E, alt. 1400 m a.s.l., leg. K. Kob, GS1818; 15 August 1998, Fai della Paganella, Andalo (TN), with F. sylvatica and P. abies, coord. 4611’ N, 1104’ E, alt. 1100 m a.s.l., leg. M. Comuzzi, GS1960; 28 August 1999, Vipiteno (BZ), with P. abies, coord. 4654’ N, 1125’E, alt. 1150 m a.s.l., leg. unknown, GS2131; 25 August 1999, Waldheim, Sarnonico (TN), with A. alba, coord. 4625’ N, 1110’ E, alt. 1230 m a.s.l., leg. G. Simonini, GS2282. FRIULI VENEZIA–GIULIA, 27 June 1998, Bosco Taggers, Lauco (UD), with P. abies, coord. 4625’ N 1257’ E, alt. 950 m a.s.l., leg. G. Zecchin, GS1899; 27 June 1998, Bosco Taggers, Lauco (UD), with P. abies, coord. 4625’ N 1257’ E, alt. 950 m a.s.l., leg. G. Zecchin, GS1900. VENETO, 10 July 1998, Val di Cadore (BL), with P. abies, coord. 4624’ N, 12 19’ E, alt. 950 m a.s.l., leg. P. Gaggio, GS1911; 11 July 1998, Val di Cadore (BL), with P. abies, coord. 4624’ N, 12 19’ E, alt. 950 m a.s.l., leg. P. Gaggio, GS1912; 11 July 1998, Val di Cadore (BL), with P. abies, coord. 4624’ N, 1218’ E, alt. 920 m a.s.l., leg. P. Gaggio, GS1913; 12 July 1998, Val di Cadore (BL), with P. abies, coord. 4624’N, 1219’E, alt. 930 m a.s.l., leg. P. Gaggio, GS1917; 12 July 1998, Val di Cadore (BL), with P. abies, coord. 4625’ N, 1218’ E, alt. 750 m a.s.l., leg. P. Gaggio, GS1918; 21 August 2012, Padola (BL), with A. alba and F. sylvatica, coord. 4635’ N, 1228’ E, alt. 1550 m a.s.l., leg. A. Testoni, GS10097. RUSSIA – 19 August 2009, Arkhys, Teberda Reserve, valley of Kyzgich river, Karachay–Cherkessia Republic, Caucasus, with Abies nordmanniana. and F. sylvatica, coord. 4340’ N, 4153’E, alt. 1050 m a.s.l., leg. T. Svetasheva, A. Kovalenko, GS10315 and GS10316. GenBank number ITS:KY677920.
Notes: During mycological surveys and mapping of Northeast of Sicily, in the territory between mountain ranges of Nebrodi, Peloritani and Madonie and the massif of Mount Etna, several collections of a Boletaceae apparently close to Rubroboletus rubrosanguineus (Cheype) Kuan Zhao & Zhu L. Yang of Northern Italy were observed. They grew exclusively with broadleaved trees and in the first instance they were included in the large colour variability of R. legaliae (Pilát & Dermek) Della Maggiora & Trassin. In the meantime, other sporadic finds of the same bolete occurred in the region Calabria, in the areas of Gambarie and Sila Piccola (Vasquez 2014). However, since the first findings, it was observed that these collections differ from these two neighbouring taxa in chorological-ecological, anatomical and macroscopic characters. The sharp delineation of the new species has become clear in additional collections (Vasquez 2012, 2013), to which some microscopic and molecular differences from the other close, but different species have been later added. Based on the BLASTn results (sequences were selected based on the greatest similarity) and the recent phylogenetic study focused on Rubroboletus (Zhao et al. 2014), sequences were retrieved from GenBank number and UNITE (http: //unite.ut.ee/) databases for the comparative phylogenetic analysis with the newly generated sequences. Caloboletus calopus (HM347645) was chosen as an outgroup species. Phylogenetic analyses were performed using the Bayesian Inference (BI) (MrBayes v. 3.2.2, Ronquist et al. 2012) and maximum likelihood (ML) (RAxML v. 7.0.4, Stamatakis 2006; RAxML version 8, Stamatakis 2014) approaches. The ITS data set comprised 52 taxa (24 from GenBank number and 14 from UNITE). BI and ML trees were congruent and only the Bayesian tree with both BYPP (Bayesian Posterior Probabilities) and MLB (Maximum Likelihood Bootstrap) values. In the analysis, the six sequences of R. demonensis form a highly-supported clade (BYPP = 1, MLB = 100); it resulted as a distinct species, sister (with low support, BYPP = 89, MLB = 54) to R. eastwoodiae. Distinctive features of R. demonensis are the overall purple blood red colour (general appearance close to R. rubrosanguineus with brighter colours), combined with frequent orange-yellow collar in the upper part of the stipe, growth with broadleaved trees (Quercus, Castanea, Fagus) and spores narrower than in the closer species, with a higher Q ratio (13.1 ± 0.5 × 4.9 ± 0.2 µm, Qm = 2.68 ± 0.11). As in the most of the boletes, red colours can be in some situations attenuate: this originates xanthoid aspect, whose appearance is quite close to R. legaliae. R. legaliae has a paler, whitish, greyish-cream pileus tending to pink or reddish [R. legaliae f. spinarii (Hlaváček) Mikšík], more velutinous, and shares in many occurrences in Sicily the same environment of R. demonensis. It has also shorter but stouter in average spores (12.5 ± 0.4 × 5.5 ± 0.1 µm, Q = 2.29 ± 0.05) (533/13/13) and generally paler yellowish-orange pores, even if some bright red pores form do exist. It grows with broadleaved trees, mainly with oaks, in neutral-acidocline or basicline soils (Lannoy and Estades 2001; Estades and Lannoy 2004; Muñoz 2005; Andersson 2013; Halama 2016). It is nevertheless much more widely distributed in Europe and its areal extends North to Denmark, East to Penza region (Russia), South to Sicily (Italy) and West to Baixo Alentejo (Portugal), including: Austria, Bulgaria, Corse, Croatia, Czech Republic, Denmark, France, Germany, Great Britain, Hungary, Ireland, Italy, Montenegro, Norway, Portugal, Romania, Russia, Sardinia, Serbia, Sicily, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey (Courtecuisse and Duhem 2011; Halama 2016). Its occurrence in Russia, until now has been overlooked (Courtecuisse and Duhem 2011; Halama 2016), but this species is known in Russia as rather rare but typical for the middle and southern oakeries, associated with Quercus robur in regions of Penza, Tula (Svetasheva 2010), Volgograd, Stavropol, Lipetsk (Sarycheva and Svetasheva, 2015). R. rubrosanguineus is a rare bolete growing in submontane and mountainous regions, on alkaline soil, in spruce and firs (Abies, Picea) woods, also mixed with beech (Fagus), rarely in pure Fagus woods and if so in the areas formerly populated by Abies; it has pileus colour more purplish than R. demonensis, without so intensive and bright red tones (Walty 1969; Cheype 1983; Lannoy and Estades 2001; Estades and Lannoy 2004; Muñoz 2005). It is widespread along the Alpine arch but its distribution western limit is constituted by Spain (Palazon 2006) and reaches the most northern distribution in the West Carpathians (Beskydy Mountains, Czech Republic), even if a doubtful record could set this limit to Belgium (Noordeloos 2010); at East, it extends to Russian Caucasus (Kiyashko 2012; Svetasheva and Kovalenko 2013). The southern limit seems to be constituted by the Italian Appennini chain, in Emilia-Romagna region, where it grows with Fagus in the areas earlier populated by Abies alba. So, its distribution includes Austria, Belgium, Bosnia and Hercegovina, Bulgaria, Croatia, Czech Republic, France, Germany, Greece, Italy, Monte Negro, Poland, western part of Russia, Slovakia, Slovenia, Spain, Switzerland (Courtecuisse and Duhem 2011). R. rubrosanguineus presents spores as slender as those of R. demonensis, but longer and broader (14 ± 0.8 × 5.4 ± 0.3 µm, Qm = 2.58 ± 0.12, (618/20/20).
From both R. rubrosanguineus and R. legaliae, R. demonensis can be distinguished by the net (reticulum) with larger meshes and thicker ribs. R. rubrosanguineus has also significantly broader pileipellis terminal elements (47.7 ± 4.9 × 7.5 ± 0.5 µm, Qm = 6.73 ± 0.49 (42/4/4)) with respect to the other two boletes (37.9 ± 5 × 5.5 ± 0.4 µm, Qm = 7.24 ± 0.89 (138/5/5) and 32.8 ± 0.1 × 5.7 ± 0., 5 µm Qm = 6.15 ± 0.51 (49/2/2) respectively for R. demonensis and R. legaliae). R. pulcherrimus (Thiers & Halling) D. Arora, N. Siegel & J.L. Frank is a North-American species apparently very similar, growing in in the North Western mixed forests of the Coastal Chain (Arora et al. 1999; Desjardin et al. 2015); it has much larger spores (14.3 ± 1.1 × 5.8 ± 0.1 µm, Q = 2.48 ± 0.15 (106/4/4) (Arora et al. 1999).
The phylogenetically closest related Boletus eastwoodiae (Murrill) Sacc. & Trotter differs in the pale greyish pileus, becoming olive-buff-grey and developing pink tones especially along the margin. The stipe is massive, abruptly bulbous with a ventricose base, with pinkish-grey colour and a concolorous or vinaceous reticulation (Bessette et al. 2000; Desjardin et al. 2015). Due to the molecular evidence offered in our work, Suillellus eastwoodiae Murrill is here combined in the genus Rubroboletus.
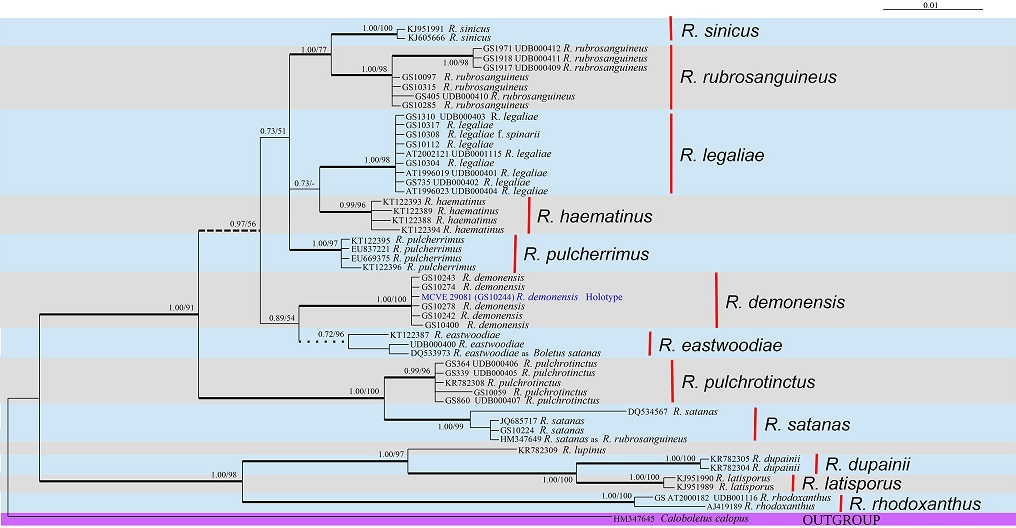
Phylogeny of the genus Rubroboletus based on a Bayesian and Maximum Likelihood Inference analysis of a ITS dataset. Bayesian posterior probability values (in bold) ≥ 0.7 and maximum likelihood bootstrap (ML) values ≥ 50% are shown on the branches. Thickened branches indicate BYPP ≥ 0.95 and ML support ≥ 70%. Dashed branches indicate BYPP ≥ 0.95 and ML bootstrap support < 70%. Nodes that receive BYPP < 0.95 but with ≥ 70% ML are indicated by small black-filled squares. Newly sequenced collections are in bold. Caloboletus calopus (HM347645) was chosen as the outgroup taxon.

Rubroboletus demonensis (GS10244, holotype). Basidiomes from the main collections. a 9 October 2013, C.da Piano Torre (Madonie), Castelbuono (PA), with Quercus pubescens s.l. and Q. ilex. b 5 September 2013, Portella dell’Obolo (Nebrodi), Capizzi (ME), with Q. pubescens s.l. c 4 August 2014, near Village Nimea, Komotini, Rhodope Regional Unit, with Q. petraea ssp. medwediewii. d 15 September 2013, Portella dell’Obolo (Nebrodi), Capizzi (ME), with Q. pubescens s.l. e 15 September 2013, Portella dell’Obolo (Nebrodi), Capizzi (ME), with Q. pubescens s.l. f 27 June 2009, Portella Sella Maria (Nebrodi), Cesarò (ME), with Q. pubescens and Q. virgiliana. g 30 June 2008, pizzo Inferno (Nebrodi), Floresta (ME), with Castanea sativa and Pinus nigra ssp. calabrica. h GS10274. i GS10244 (Holotype). j GS10708. k GS10278. l GS10242. Bars = 50 mm.
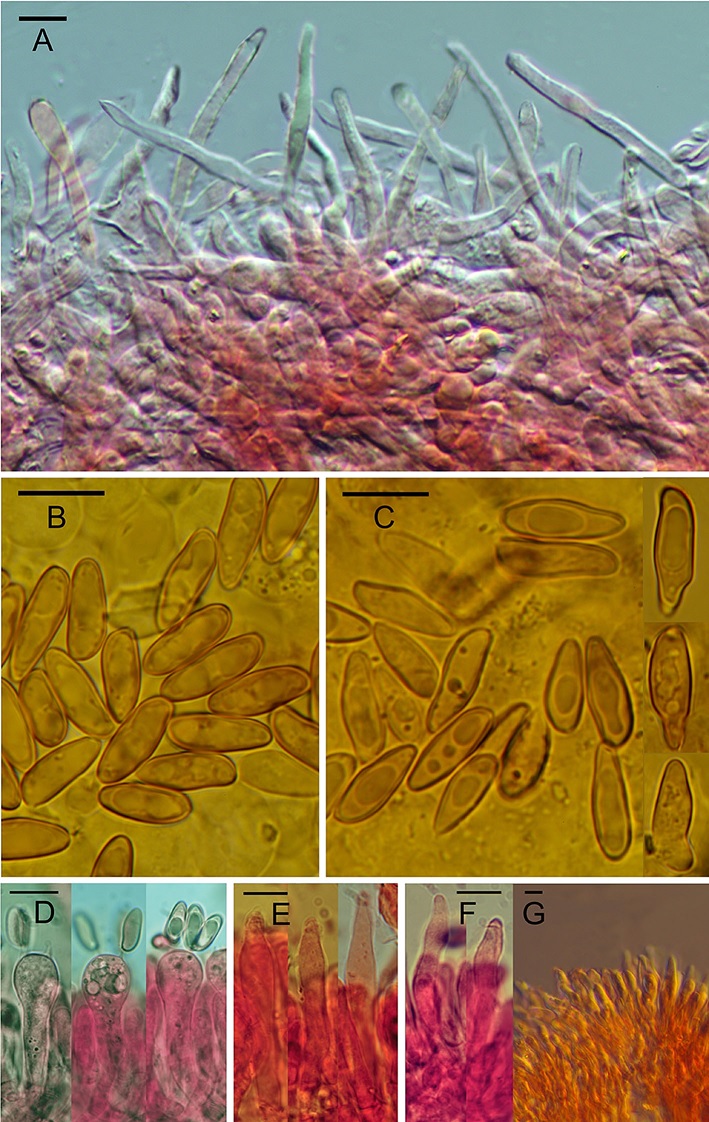
Rubroboletus demonensis (GS10244, holotype). Microscopic structures. a Pileipellis in 3% NH3+ Congo Red, with Differential Interference Contrast (DIC) (GS10244). b Spores in 3% NH3 (GS10244). c left Spores in 3% NH3 (GS10274). c right Abnormal spores in 3% NH3 (GS10243). d Basidia in 3% NH3+ Congo Red (GS10245). e Facial cystidia in 3% NH3+ Congo Red, with DIC (GS10245). f Marginal cystidia in 3% NH3+ Congo Red, with DIC (GS10245). Bars = 10 μm.
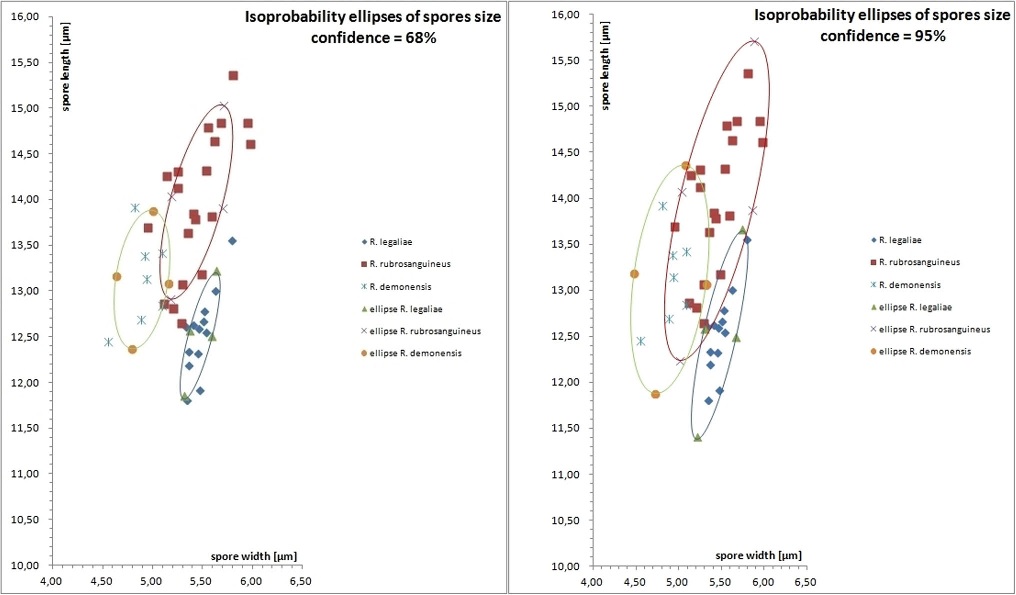
Distribution of spore size of R. demonensis (7 collections), compared with R. legaliae (13 collections) and R. rubrosanguineus (20 collections), using “isoprobability ellipses”. It is shown the distribution of the average values of spore size for any of the collections, at the confidence of 68% (corresponding to 1st deviation, left side) and at the confidence of 95% (correspondent to 2 × st. deviation, right side).
