Russula pseudoflavida A. Ghosh, Hembrom, I. Bera & Buyck
MycoBank number: MB; Index Fungorum number: IF; Facesoffungi number: FoF 11437;
Description
Pileus very small to medium-sized, 10–45 mm in diam., convex at young, becoming planoconvex to applanate, uplifted with age, centrally depressed to umbilicate with maturity, margin tuberculate striate, decurved to plane with age; cuticle smooth, velvety, viscid and shiny when wet, dull upon drying, peeling to 1/2nd of the radius, deep orange (6A–B7–8) or brownish orange (6–7C7–8) at young, then yellowish orange, orange yellow to deep yellow (4A7–8) to orange to deep orange (5A7–8). Pileus context 5–10 mm thick at the disc, thinning towards the margin, compact, brittle, chalky white (1–2A1), unchanging after bruising or cutting; turning salmon pink (6A4) with FeSO4 and and deep to dark turquoise (24E–F7–8) in guaiacol. Lamellae 10–15 mm deep, adnexed to narrowly adnate, close, crowded to very crowded (10–22/cm at pileus margin), apex rounded, chalky white (1–2A1), forked near stipe apex; lamellulae absent; edges marginate, deep orange or dark orange (5A8). Stipe 10–30 × 4–9 mm, cylindrical, central, firm and brittle; surface dry, smooth, velvety, concolorous to pileus, chalky white (1–2A1) at the stipe apex. Stipe context stuffed, chalky white (1–2A1), unchanging after bruising or cutting; turning salmon pink (6A4) with FeSO4 and and deep to dark turquoise (24E–F7–8) in guaiacol. Odour not distinctive. Taste mild. Spore print not obtained.
Basidiospores globose, broadly ellipsoid to ellipsoid, (5.5–)5.7–6.05–6.5(–7.0) × (4.4–)4.8–5.2–5.6(–6.2) μm, Q=(1–)1.11–1.17–1.22(–1.25); ornamentation amyloid, composed of obtuse, moderately large and relatively dense warts, up to 0.6 μm high, merged in short ridges, which are interconnected by numerous fine line connections; suprahilar spot amyloid, relatively large and conspicuous; apiculi up to 0.9 μm high. Basidia (18–)21–27–32(–39) × (9–)9–10–10.5(–11) μm, 4-spored, subclavate to clavate, sterigmata up to 5 μm long. Hymenial gloeocystidia on lamellae sides (39–)41.7–49–56(–60) × (7–)8–9.4–11(–12) µm, rare, clavate to subclavate with mostly rostrate (up to 13 μm long) or obtuse-rounded apex, emergent up to 15 μm above the other elements of the hymenium, few deeply embedded; near the lamellae edges usually smaller and narrower, measuring (27–)30.2–38.3–46.3(–52) × (6–)6.6–8.3–10(–11) µm, cylindrical, subclavate to clavate with rostrate or obtuse-rounded apex; contents granulose, without reaction in sulfovanillin. Subhymenium layer up to 25 µm thick, pseudoparenchymatous. Marginal cells similar to hyphal terminations in pileipellis, mainly cylindrical, measuring (12–)15.1–22.3–29.6(–35) × (3.5–)4.2–5.1–6(–6) μm, apically obtuse-rounded; mixed with occasional basidia or basidioles. Hymenophoral trama mainly composed of large nests of sphaerocytes and intermixed with hyphal elements. Pileipellis orthochromatic in Cresyl blue, sharply delimited from the underlying sphaerocytes of the context, 100–200 μm deep, two layered; vaguely divided in 70–150 μm deep suprapellis of relatively dense, composed of erect or ascending hyphal terminations, arranged in a trichodermal structure and dispersed primordial hyphae; subpellis 30–50 μm deep, composed of horizontally oriented and densely arranged pilear hyphae. Acidoresistant incrustations present. Hyphal terminations near the pileus margin flexuous, thin-walled, composed of chain of 2- to 3-celled, branched at the subterminal cells or the cells just below, sometimes incrustation present; terminal cells measuring (16–)21.5–35–48.4(–66) × (4–)5–6.4–7.7(–9.5) μm, cylindrical or slightly narrowed towards apex or ventricose or narrowly uniform, apically obtuse-rounded or acute; subterminal cells usually equally long but sometimes wider (up to 11 μm), often with lateral projections. Hyphal terminations near the pileus centre of similar structure, terminal cells slightly shorter and less wide, measuring (14–)19.1–28–37(–45) × (3–)3.5–5–6.5(–9) μm, cylindrical or slightly narrowed towards apex or ventricose or narrowly uniform, apically obtuse-rounded or acute; subterminal cells usually equally long but sometimes wider (up to 13 μm). Primordial hyphae near the pileus margin typically 2- to 3-celled, flexuous, very long, thick-walled (up to 1 μm); terminal cells (58–)65.5–111–157(–225) × (2–)2.8–3.8–4.8(–6) μm, mainly attenuate, apically mostly acute, subterminal cells long, cylindrical; usually with strong incrustations covering most of its surface. Primordial hyphae near the pileus centre 2- to 3-celled, flexuous, very long, thick-walled (up to 1 μm), slightly shorter, terminal cells (45–)46–80.6–115(–165) × (2–)3.4–4.4–5.5(–6) μm, cylindrical to attenuate, apically mostly acute; usually with strong incrustations covering most of its surface. Pileocystidia not observed. Clamp connections absent in all parts.
Material examined: India, West Bengal, Jhargram district, Tuluha, alt. 80 m, N 22°19’18” E 87°05’34”, 13 August 2020, A. Ghosh, AG 20-058 (CAL 1862, holotype!).
Sequence data: ITS: OL471685 (nrITS Holotype) and OL471686 (nrITS) LSU: ON365928 (nrLSU, holotype), ON365929 (nrLSU) mtSSU: ON387512 (mtSSU, holotype), ON387511 (mtSSU) rpb2: ON398067 (rpb2, holotype), ON398068 (rpb2)
Notes: In the field, our new species is a look-alike of R. flavida and related taxa in subsect. Auratinae Bon. However, since our specimens were associated with tropical dipterocarp trees, this habitat also suggested a possible placement in the African subsect. Testaceoaurantiacinae Buyck (see Buyck 1993). Both subsections are composed of species that have bright yellow to orange surfaces of pileus, stipe and gill edges and both harbour species that lack pileocystidia and have multicelled, often long hyphal extremities at the pileus surface. Both subsections were part of the same larger lineage, that received moderate although significant support (77% MLBS), in the multilocus phylogeny published by Buyck et al. (2018). This clade, in a rather basal position in the crown clade of subg. Russula, was essentially composed of African species from various subsections, but also comprised northern hemisphere Auratinae and the European R. romellii Maire. It is interesting to observe now that our new species is part of a northern hemisphere lineage in Russula and not of an African lineage, notwithstanding the fact that ectomycorrhizal dipterocarp trees arrived in India from Africa associated with African ectomycorrhizal fungi.
Important differences in field aspect are inexistent between American and Asian collections of R. flavida sensu lato, but these differ both greatly from the European R. aurea and the very closely related Russula aurantioflava Kiran & Khalid, reported from Pakistan (Adamčík et al. 2019). Both latter species are much less uniform in color and also more variable, with pileus colors that vary from purplish to wine red, over brick red and orange to yellow, sitting on top of a stipe that can be almost entirely white.
Our new species differs microscopically from similar species in Auratinae in the smaller size of its basidiospores, although available data reflect few measured specimens. North American R. flavida Frost has basidiospores measuring (7.1–)7.6–7.9–8.3(–8.6) × (5.8–)6–6.4–6.7(–7) μm for the holotype (Adamčík et al. 2018), but these were reported as 5.5-8.5(9.6) x 5-7 um in Bills & Miller based on different collections (1984). Russula aurantioflava has a spore size of (8–)8.5–9.6–10.7(–11.84) × (6.2–)7.4–8.5–9.5(–10.6) μm.
The new R. pseudoflavida is considerably different from the above species in its association with dipterocarps. There are also some puzzling ITS sequence similarities observed between collections made, possibly associated with dipterocarps, in the north-eastern part of Thailand (MW468068, MW468067) showing 100% similarity (for 100% coverage) with a first North American ITS sequence (EU598170), but only 95% (for 100% coverage) for a second ITS, both obtained from R. flavida collections during the same field trip in the Smoky Mountains, Tennessee. Host specificity for other Auratinae seems not very high. The well-documented R. aurea has a distribution that extends from Mediterranean climates all the way into the colder parts of Europe, and occurs under various deciduous trees and conifers and on various types of soil (Sarnari 2005). Russula flavida is found in mixed forests with various Quercus, Betula, but also conifers (Bills & Miller 1984). Russula aurantioflava was originally reported as ectomycorrhizal with conifers (Adamčík et al. 2019). However, based on 100% similarity top scores in BLAST for ITS sequence deposits MN7048154 and MN704815 in GenBank, it occurs also in the very north-eastern part of China (Xing et al. 2020) in forests dominated by Quercus mongolica (98%) with intrusion of Betula platyphylla Sukaczev (2%), resulting finally in a very similar host range as for R. flavida.
For R. aurea, the pileipellis has always been interpreted as devoid of any well-defined pileocystidia or primordial hyphae, and this is also true for the quite similar R. aurantioflava. However, for American and Asian specimens of R. flavida sensu lato, the question of absence/presence of well-defined pileocystidia or primordial hyphae is a difficult one and there exist contradictory observations. The whole pileipellis is very distinctly incrusted with yellow pigment which makes it probably difficult to interpret the presence of authentic primordial hyphae; also presence of pileocystidia is difficult to ascertain because they do not react with sulfovanillin.
The sister clade for Auratinae was for the first time suggested in a multilocus phylogeny presented by Adamčík et al. (2019) with the description of R. wielangtae G.M. Gates, Caboň & Jančovič. from Tasmania, to which their ITS-based phylogeny added also R. atroviridis Buyck from New Zealand and the hypogeous R. theodoroui (T. Lebel) T. Lebel from Australia.
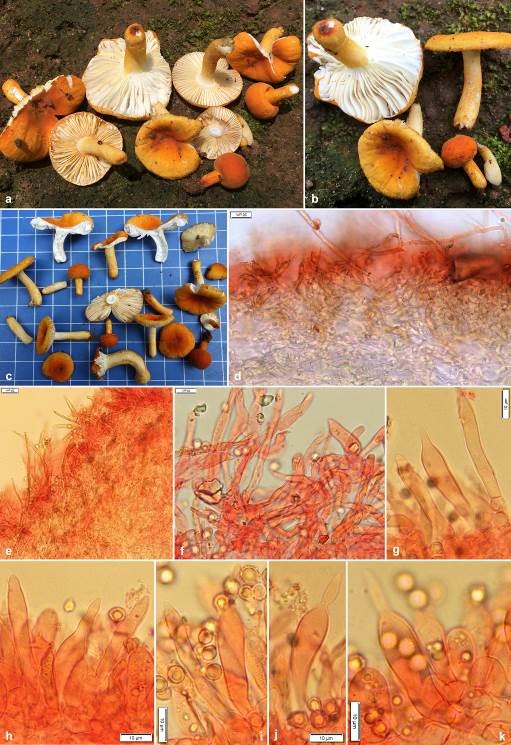
Figures 1. Russula pseudoflavida (CAL 1862, holotype) a–c Fresh and dissected basidiomata in the field and basecamp. d−f Transverse section through pileipellis showing elements. g Transverse section through lamellae showing hymenial gloeocystidia near the lamellae edges. h−k Transverse section through lamellae showing hymenial gloeocystidia near the lamellae sides. Scale bars: d, e = 20 μm; f−k = 10 μm.
