Resinoscypha T. Kosonen, Huhtinen & K. Hansen Fig. 14e, 19, 20
MycoBank number: MB 835739; Index Fungorum number: IF 835739; Facesoffungi number: FoF 14072;
Etymology: Referring to the cyanophilous resinous substance inside the hairs.
Type species: Resinoscypha variepilosa (R. Galán & Raitv.) T. Kosonen, Huhtinen & K. Hansen.
Included species: R. monoseptata, R. variepilosa.
A light-coloured, hairy, lignicolous genus characterised by long, smooth, hyaline, thin-walled, regularly somewhat flexuous, cylindrical to narrowly conical hairs with 1– 4 septa, tapering towards a blunt apex and showing cyanophilous, non-glassy resinous substance especially inside, shorter marginal hairs very variable in shape, refractive globules lacking. Excipulum of textura angularis–prismatica, without dextrinoid reactions, but both hairs and excipular cells may contain strongly amyloid nodules. Asci arising from croziers, apical pore MLZ+, 8-spored. Ascospores ellipsoid to oblong-ellipsoid, aseptate to 1-septate. Paraphyses cylindrical.
Notes: — Emended descriptions of both included species were provided by Huhtinen (1993b) and also earlier by Huhtinen (1987b) and are therefore not reproduced here, but new additional illustrations are provided (Fig. 14e, 19, 20). Resinoscypha is phylogenetically a distinct genus, sister to all other taxa in the Hyaloscyphaceae s.str. clade (Fig. 2). The two species, originally described in Protounguicularia by Raitviir & Galán (1986), were separated from the type species, P. brevicapitata (now Olla transiens), mainly based on the notable differences in hair inclusions (Huhtinen 1987a). In O. transiens the material inside the hairs is glassy and strongly dextrinoid (Fig. 14c). The true glassiness is verified by observations in CB, where the colour does not penetrate the glassy substance. The two Resino scypha species have resinous material concentrated at the apex of the hairs and at septal areas (Fig. 14e, 19a, e, 20a, e). These inclusions are MLZ- and strongly CB+. The taxonomic value of these differences, as well as the differences in ascal development (arising from simple septa in the type species versus from clear croziers in the others), passed largely unnoticed by the original authors. The characteristic CB+ resin inside the hairs, combined with the variably shaped and septate hairs, delimit this new genus from Eupezizella and Mimicoscypha, which both may have a variable number of amyloid nodules in the excipula.
When combining the two species to Arachnopeziza, Huhtinen (1987a) emphasized the cylindrical septate hairs. It was suggested, however, that these species occupy ‘a transitional position’ between Arachnopezizoideae and Hyaloscyphaceae and the author contemplated on alternative solutions (e.g., a separate genus).
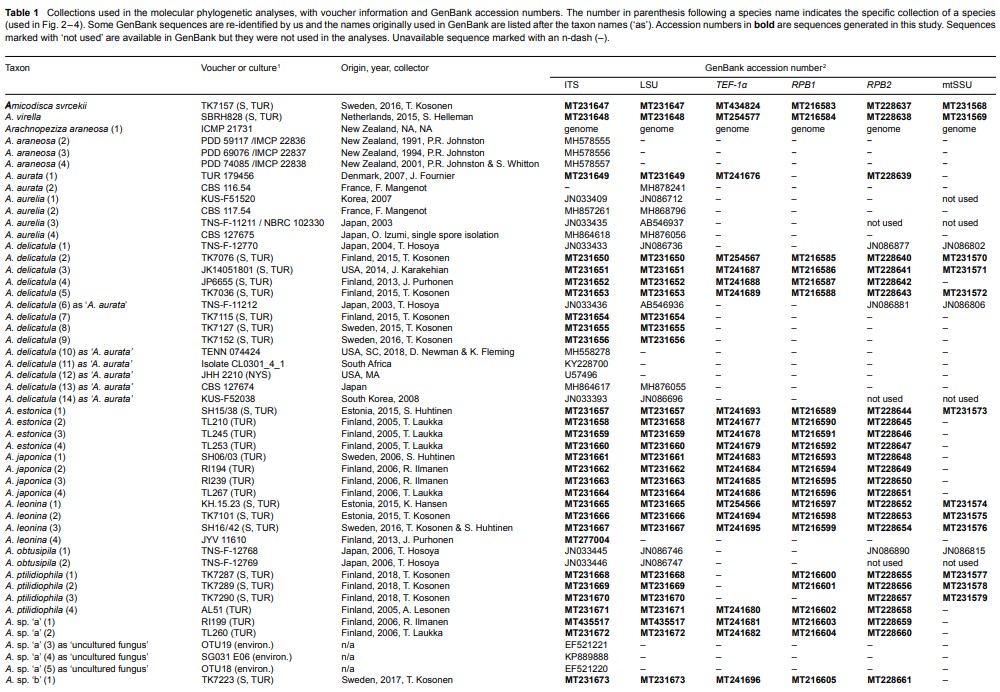
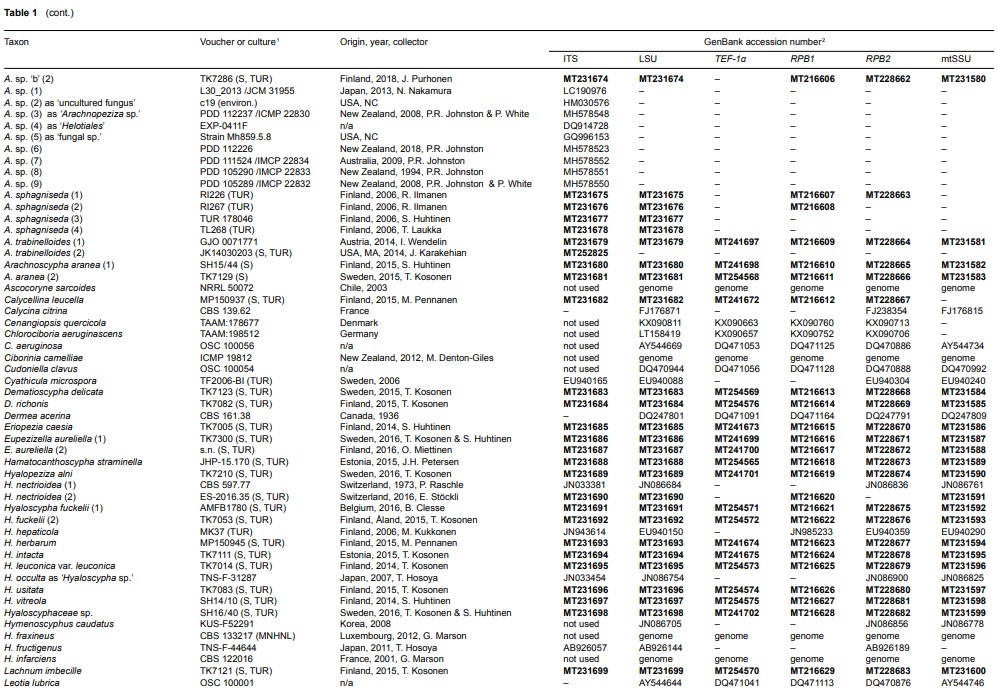
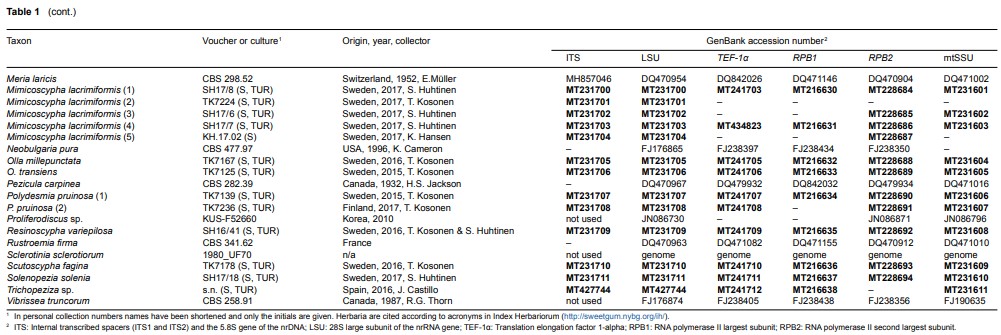
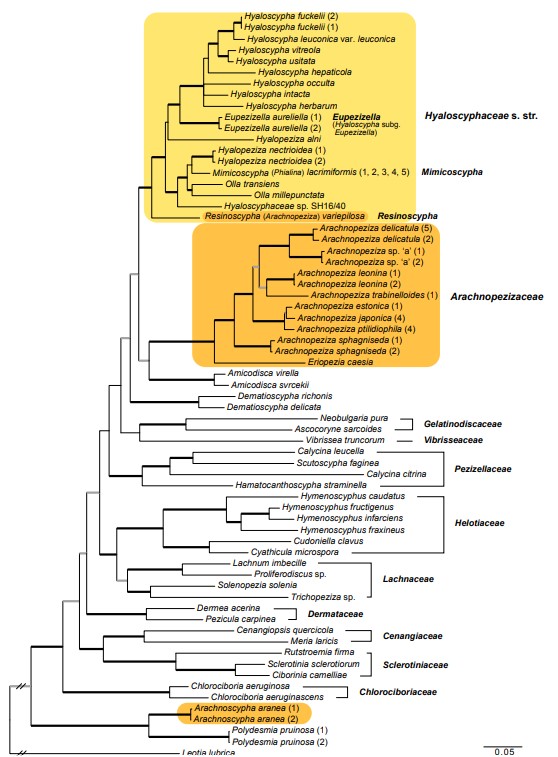
Fig. 2 Phylogenetic relationships of Arachnopezizaceae (orange square) and Hyaloscyphaceae (yellow square) among members of Helotiales based on Bayesian analyses of combined LSU, RPB1, RPB2, TEF-1α and mtSSU loci. Arachnoscypha aranea and Resinoscypha variepilosa (orange squares) were previously treated in Arachnopezizaceae. Thick black branches received both Bayesian posterior probabilities (PP) ≥ 0.95 and maximum likelihood bootstrap value (ML-BP) ≥ 75 %. Thick grey branches received support by either PP ≥ 0.95 or ML-BP ≥ 75 %. The number in parenthesis after a species name refers to an exact collection (see Table 1).
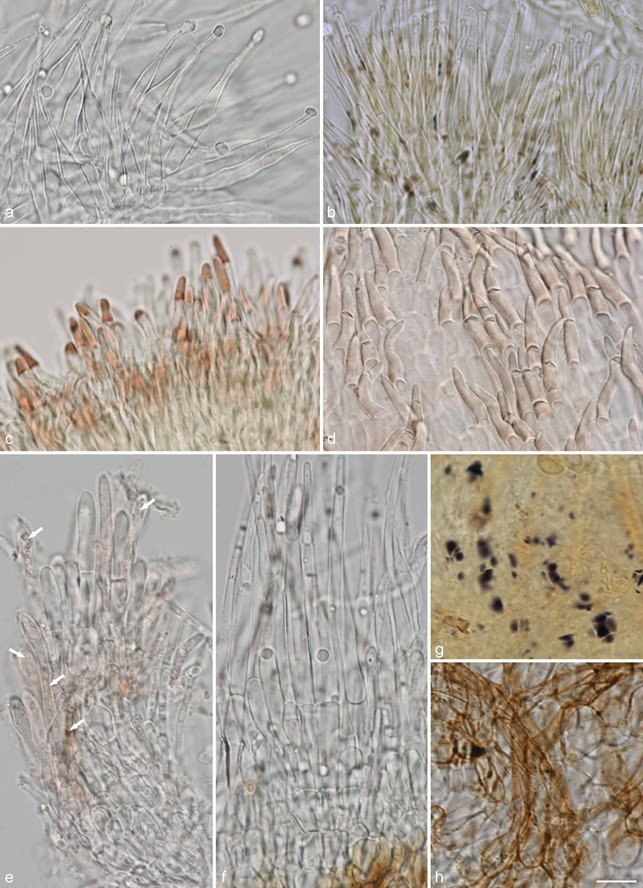
Fig. 14 Examples of hair shapes, inclusions and reactions, and of amyloid nodules in excipulum cells, in Hyaloscyphaceae. a. Thin-walled aseptate hairs,
Hyaloscypha spiralis (in water); b. hairs showing amyloid and dextrinoid nodules, Eupezizella aureliella (in MLZ); c– d. dextrinoid glassy hairs in Olla (in MLZ):
c. Olla transiens; d. Olla millepunctata; e. septate hairs showing resinous matter inside the hairs (arrows), Resinoscypha variepilosa (in water); f– h. Mimico scypha lacrimiformis: f. septate hairs with refractive globules; g. purple nodules in ectal excipulum cells (in MLZ); h. bulbous basal cells of hairs with exudates (in water) (a: KH.16.02; b: T. Kosonen 7296; c: U. Söderholm 4829; d: T. Kosonen 7155; e: S. Huhtinen 16/41; f– h. KH.17.02). — Scale bars = 10 µm. — All from fresh material. — Photos: a, e– h. K. Hansen; b– d. T. Kosonen.
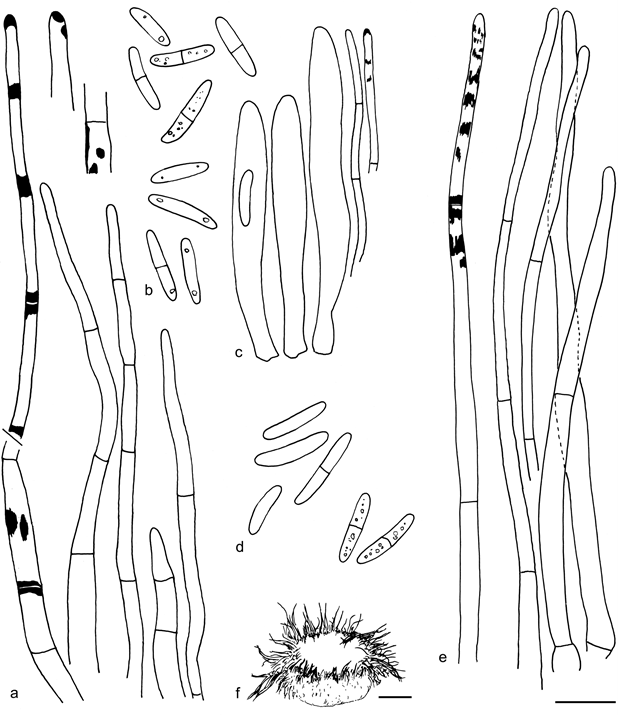
Fig. 19 Resinoscypha monoseptata. a. Marginal hairs in lactic acid, leftmost in CB showing the CB+ resinous substance, the two details in MLZ showing deep amyloid nodules; b. spores, upper five in CB, lower four in lactic acid; c. asci and paraphyses in lactic acid, paraphyse on right in CB showing the scanty resinous substance; d. spores, upper four in CB, lower two in MLZ; e. marginal hairs in lactic acid, leftmost in CB showing the CB+ resin; f. dry apothecium (a – c: isotype, Herb. Galán 6154; d– f: CUP 59279). — Scale bars: a– e = 10 µm, f = 100 µm. — Drawings: S. Huhtinen.
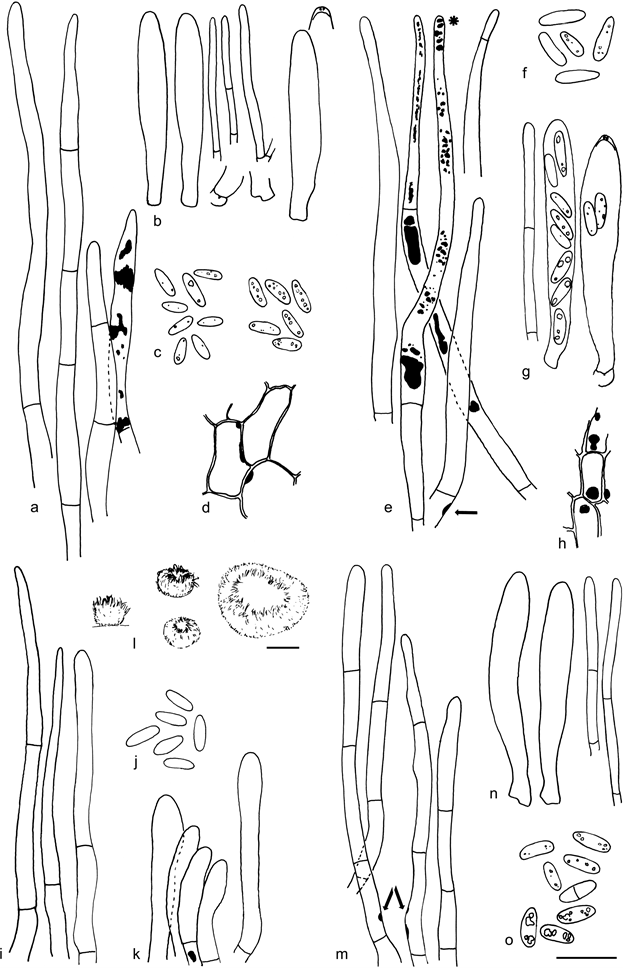
Fig. 20 Resinoscypha variepilosa. a. Marginal hairs in CR, rightmost in CB showing the CB+ resinous contents; b. asci and paraphyses; c. spores in CB (left) and CR (right); d. ectal excipulum showing the amyloid nodules; e. marginal hairs showing the brown resinous substance inside, in MLZ, hair with asterisk (*) in water, one hair with basal amyloid nodule (arrow); f. spores in MLZ; g. asci and paraphysis; h. ectal excipulum showing the amyloid nodules; i. marginal hairs in CR, rightmost hair in water; j. spores in CR; k. short marginal hairs in MLZ showing one rare amyloid nodule; l. dry apothecia; m. marginal hairs in MLZ, some showing the amyloid nodules (arrows); n. asci and paraphyses; o. spores in CB, three lower in KOH (a – d: holotype; e– h: S. Huhtinen 87/131; i– k: S. Huhtinen 16/41; l– o: S. Huhtinen 86/60). — Scale bars: a– k, m– o = 10 µm, l = 100 µm. — Drawings: S. Huhtinen.
Species
