Pallidocercospora acaciigena (Crous & M.J. Wingf.) Crous & M.J. Wingf. Stud. Mycol. 75: 74 (2012)
≡ Mycosphaerella acaciigena Crous & M.J. Wingf., in Crous, Groenewald, Pongpanich, Himaman, Arzanlou & Wingfield, Stud. Mycol. 50(2): 463 (2004)
Index Fungorum number : IF 564821 ,Facesoffung inumber: FoF 00439; Fig. 1
Saprobic on palms. Sexual morph Ascomata 50 – 70 μm high., scattered, solitary to gregarious, immersed to semi-immersed, with protruding papilla, visible as black spots on host surface, uniloculate, globose to subglobose, glabrous, ostiole central, with minute papilla, obtuse at the apex. Peridium 4 – 10 μm wide, thin-walled, of equal thickness, comprising 2 – 6 layers of brown pseudoparenchymatous cells, arranged in textura angularis. Hamathecium lacking pseudoparaphyses. Asci (27–) 30 – 40 (–53) × (5–) 6 – 8 (–9) μm (x̄ = 36.6 × 7.2 μm, n = 25), 8 – spored, bitunicate, fissitunicate, cylindric-clavate to ampulliform, or obclavate, often ventricose, apedicellate, apically rounded, with an ocular chamber (0.5 – 1 μm wide). Ascospores 9 – 12 × 2 – 3 μm (x̄ = 10.5 × 2.9 μm, n = 30), overlapping 2 – 3-seriate, oblong to clavate, hyaline, 1 – septum, not contricted to slightly constricted at the septa, with smooth or rough walls, with small guttules, or slightly echinulate, mostly upper cell wider than lower cell. Asexual morph Undetermined.
Culture characters – Colonies on MEA slow growing, reaching 18 – 20 mm diam. after 3 weeks at 25–30 °C, dark greenish at the margin, pale greenish at the centre, becoming pale greenish-grey to grey at the centre; reverse dark greenish, becoming paler in the centre; dense, irregular, raised to umbonate, with entire edge, velvety, slightly radiating, curved, radially furrowed.
Material examined – THAILAND: Chiang Rai, Muang District, Khun Korn Waterfall, on dead leaves of palm, 12 May 2010, R. Phookamsak RP0032 (MFLU 11–0153), living culture, MFLUCC 10–0561. GenBank ITS: KP744451; LSU:KP744494.
Notes – Pallidocercospora was introduced by Crous et al. (2013) to accommodate taxa that are not congeneric with Mycosphaerella (Crous et al. 2012). The genus was considered to be distinguished from Mycosphaerella based on its asexual morph. Mycosphaerella is represented by Ramularia asexual morphs (Quaedvlieg et al. 2013), whereas, Pallidocercospora has pseudocercospora-like asexual morphs (Crous et al. 2004, 2012). Pallidocercospora acaciigena is morphologically similar to Mycosphaerella thailandica Crous et al., but differs in its asexual morph and ascomata (Crous et al. 2004). Pallidocercospora acaciigena has longer pseudocercospora-like conidia than M. thailandica and the ascomata of P. acaciigena are also dense, superficial and clustered (Crous et al. 2004). Pallidocercospora acaciigena has been reported as pathogen on Acacia in Venezuela (Crous et al. 2004), while our isolate was found as a saprobe on palms in Thailand, representing a new host and continent. Based on a megablast nucleotide search in GenBank of ITS gene, our isolate is related to P. acaciigena (99 % similarity), P.crystallina (99 %), and Mycosphaerella heimii (99 %). However, the ITS pairwise comparison has shown that our isolate is most closely related to P. acaciigena. Phylogenetic analyses of LSU genes shows that our isolate forms a strongly supported clade with P. acaciigena.
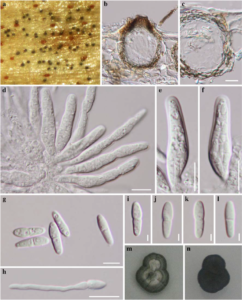
Fig. 1 Pallidocercospora acaciigena (MFLU 11–0153) a Ascomata visible as black spots on host surface b Vertical section through an ascoma c Peridium d, e, f Asci g, i, j, k, l Ascospores h Germinating ascospore m, n Culture characters. Scale bars: b = 20 μm, c – f = 10 μm, g, h, = 5 μm, i – l = 2 μm.
