Nigrospora vesicularifera M. Raza & L. Cai, Fungal Diversity 99(1), 1–104 (2019)
Index Fungorum number: IF 556686; Facesoffungi number: FoF 06175; Fig. 75
Saprobic on the dead leaf of Litchi chinensis. Sexual morph: Undetermined. Asexual morph: Hyphomycetous. Colonies on the natural substrate black to brown. Mycelium composed of smooth, branched, septate, and rarely brown to hyaline hyphae. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 7.5–10 × 12.5–15.5 μm (x̅ = 8.7 × 13.9 μm, n = 20), monoblastic without proliferation, solitary, pale brown, discrete, determinate, short ampulliform, with liberated base and hyaline vesicles, delimiting the conidia from conidiogenous cells. Conidia 11.5–19 × 13–17 μm (x̅ = 15.25 × 15 μm, n = 20), solitary, globose or subglobose, black, shiny, smooth, aseptate.
Culture characteristics – Colonies on PDA fast-growing, reaching 7.2 cm diam. after eight days of incubation at 25 ºC fast-growing, covering the petri dish in three weeks. Colony circular, entire to filiform margin, medium sparse, flat with fimbriate edge, downy, aerial mycelium, rough surface, colony from above initially white, turning dark grey when mature, and reverse initially white, and produces dark brown to black pigment in PDA when mature.
Material examined – Thailand, Chiang Rai Province, Nang Lae, a dead leaf of Litchi chinensis, 20 June 2021, NP Samaradiwakara NPSC05 (MFLU 22-0081), living culture MFLUCC 22-0014.
GenBank accession numbers – ITS: ON211313, tef1-α: ON622464, β-tubulin: ON622465.
Known distribution (based on molecular data) – China (Raza et al. 2019), Thailand (this study).
Known hosts (based on molecular data) – Saccharum officinarum (Raza et al. 2019), Litchi chinensis (this study).
Notes – Nigrospora species are cosmopolitan in distribution and associated with a wide range of crops of economic significance. They comprise endophytes, pathogens, and saprobes (Sun et al. 2011, Rashmi et al. 2019). Raza et al. (2019) recently erected Nigrospora vesicularifera to accommodate six pathogenic strains isolated from sugarcane in China. Nigrospora vesicularifera is distinguished by vesicles surrounded by the septum between its conidiogenous cells and conidia (Raza et al. 2019). Even though the presence of a vesicle is relevant to N. musae, N. sphaerica, and N. vesicularis, those three species are phylogenetically distant from N. vesicularifera. The multi-gene (ITS, tef1-α, and β-tubulin) phylogenetic analyses revealed that our strain clusters within the N. vesicularifera clade with 85% maximum likelihood bootstrap support and 0.98 Bayesian posterior probability (Fig. 2). This is the first record of N. vesicularifera as a saprobe on dead leaves of Litchi chinensis and the first geographical record of N. vesicularifera in Thailand.
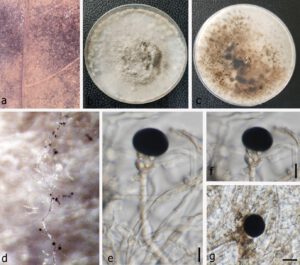
Fig. 1 – Nigrospora vesicularifera (MFLU 22-0081, a new geographical and host record). a Appearance of fungal colonies on host substrate. b–c Colony on PDA (b from above, c from below). d Sporulation on PDA medium. e–f Conidiogenous cells. g Conidia. Scale bars: e–g = 10 μm.

Fig. 2 – Phylogram generated from maximum likelihood analysis based on the combined ITS, β-tubulin and tef-1α sequence data of Nigrospora. Ninety-three strains of Nigrospora are included in the combined analyses, which comprised 1164 characters (444 characters for ITS, 384 characters for β-tubulin, 334 characters for tef-1α). The RAxML analysis yielded the best scoring tree, which was used as the backbone tree, with a final likelihood value of -8177.485492. The matrix had 526 distinct alignment patterns with 7.86% of undetermined characters or gaps. Under the Akaike Information Criterion (AIC) the evolutionary model HKY+G is applied to ITS and β-tubulin genes, and GTR+I+G is applied to tef-1α gene. Maximum likelihood (ML) with bootstrap support greater than or equal to 50% and Bayesian posterior probabilities (PP) values greater than or equal to 0.8 are given near nodes, respectively. The tree is rooted with Apiospora vietnamensis (IMI 99670) and A. sichuanensis (HKAS 107008). Ex-type strains are in bold and the species described herein are denoted in yellow. Hyphen (-) represents support values below 50% (ML) and below 0.80 (PP).
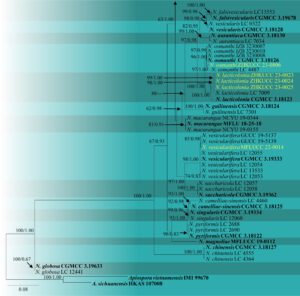
Fig. 2 – Continued.
