Watsoniomyces obsoletus (Nyl.) D. Hawksw., M. Powell & T.Sprib., comb. nov.
MycoBank number: MB 558105; Index Fungorum number: IF 558105; Facesoffungi number: FoF;
Basionym: Lecidea obsoleta Nyl., Flora, Regensb. 48: 604 (1865).
Synonyms: Biatorina obsoleta (Nyl.) Arnold, Flora, Regensb. 53: 474 (1870). Thrombium cretaceum W. Wats., J. Bot. Lond. 71: 337 (1933) [“1932”]. Lecidea watsonii P. James, Lichenologist 3: 98 (1965); nom. nov. for Thrombium cretaceum W. Wats. Non Thrombium cretaceum Oxner, Bot. Zhurn., Kiev 12(2): 93 (1955). Non Lecidea cretacea (Müll. Arg.) Hue, Nouv. Arch. Mus. Hist. Nat. Paris, se´r. 5, 4: 11 (1914).
Types: UK: Sussex: nr Lewes, “ad cretam”, Jones (holotype of Lecidea obsoleta e not traced); Buckinghamshire: Cliveden, Cliveden Gardens, on chalk fragments forming a loose scree in shrubby woodland sloping steeply downwards towards the River Thames, 41(SU)/9089.8398, 2 Aug. 2016, M. Powell (K(M) 255529 e neotype designated here for Lecidea obsoleta, IF 558106; BM 001242126, ALTA, e isoneotypes); Sussex, West: East Dean, on small chalk stones in scrub on Breendown, Aug. 1913, W. Watson [as “Thrombium cretaceum mihi ad interim”] (BM 000975501 e holo- type of T. cretaceum) (Fig. 3A-F).
Description: Thallus inconspicuous, thin, effectively endolithic, the chalk becoming crumbly and minutely granular where algal or cyanobacterial cells and hyphae are present; surface subgelatinous, with an orange hue from included algal cells evident when fresh or moistened; delimiting prothallus absent. Ascomata partially immersed to sessile, at first appearing perithecioid, subglobose, mainly 100e175 mm diam, finally expand into a ± flat apothecioid disc, 0.1-0.2 (-0.35) mm diam; thalline exciple absent; true exciple persistent, pale orange-brown in section, more densely pigmented near the upper (outer) edge, with angular crystalline inclusions, becoming somewhat dentate; epithecium orange-brown to red- brown, Ke; hymenium colourless or pale straw, c. 120e130 (e150) mm tall, hymenial gel KI þ blue (particularly marked in Lugol’s iodine solution); hypothecium c. 10 mm deep, colourless to pale orange-brown. Interascal filaments long and slender, some- what sinuous, mostly unbranched but sometimes sparsely branched near the base, and anastomosed if stained and squashed, extending above the tops of the asci, and there often apparently clumped together in bundles to form the epithecium, 1.5e2 mm wide, apices not markedly swollen or capitate. Asci 50-70 x (11-) 12-14 mm, subcylindrical to elongate obpyriform, somewhat attenuated towards the apices, apices somewhat thickened but lacking any special apical apparatus, two-layered, the inner walls 0.5-1 mm thick and K/I e, and the outer gelatinous 1-1.5 mm thick and K/I blue, after ink-vinegar treatment with a “pleated-cellophane” appearance, fragile, and breaking off at the top and bottom to produce sleeve-like cylinders, 8-spored. Ascospores at first uni- seriately arrange in the asci but becoming overlapping as they mature, narrowly ellipsoid to broadly fusiform, sometimes some- what attenuated at one end giving a more lemoniform appearance, colourless, simple, smooth, walls ca 1 mm thick, containing conspicuous inclusions (perhaps oil drops) of various sizes which persist even after the addition of K or N, contents Iþ somewhat
Photosynthetic partner: There are often assorted photosynthetic organisms associated with this species, and indeed Watson (1933) noted that cyanobacteria were “sometimes present” and that trentepohlioid algae “sometimes occur as well”. We have not investigated the photosynthetic partners in detail, but from microscopical observations the predominant one appears to be a cyanobacterium belonging to Scytonema. We also noted algae with orange-pigmented chloroplasts (cf. Trentepohlia), and also clusters of green algal cells showing binary division (cf. Chlorella).yellow-orange, (12-)16-19 x (5e)6-8 mm. Conidiomata not seen.
Ecology: On chalk pebbles in disturbed habitats, particularly around the entrance to rabbit burrows, or where the ground has been scraped to expose chalk fragments on the surface, but also on chalk fragments embedded in footpaths; only on pebbles under 10 cm across at the Cliveden site. Part of a distinctive assemblage with various verrucarioid species, and termed the Lecideetum watsoniae by James et al. (1976) who included releve´ data from stands in Hertfordshire, Norfolk, Surrey and Sussex.
Distribution: Known with certainty only from southern and eastern England (Bedfordshire, Buckinghamshire, Cambridgeshire, Hertfordshire, Isle of Wight, Norfolk, Surrey, Sussex, and Yorkshire). The species also appears to have been lost from several localities in southern England over the last 50 y as a result of a reduction of rabbit grazing pressure and subsequent encroachment of scrub.
The conservation evaluation of British lichens categorizes the species as of “least concern” and “nationally scarce”, but one for which the UK has “international responsibility” for conservation as a probable endemic (Woods and Coppins 2012). We consider it probable, however, that the species occurs on chalk on the French side of the English Channel, but it has not so far been reported from there or any other part of France.
The identity of records of L. lichenicola from Ukraine is uncertain. Smerechinska (2005) reported L. lichenicola and provided ascospore measurements consistent with that of the British material. She also corrected elements of the description of T. cretaceum Oxner, but did not state explicitly whether she felt that species was conspecific with L. lichenicola. Later, Kondratyuk & Roms (2010) listed T. cretaceum Oxner as a synonym of L. lichenicola, although the original description of the former clearly depicts a perithecial section (Oxner 1955). Material from Ukraine was not available for us to review for this study.
Notes: More apparent and distinctive when wet as then the thallus becomes subgelatinous and the ascomata stand out from this as reddish brown translucent dots. On drying, the ascomata are much less conspicuous, appearing only as dark specks through a hand-lens.
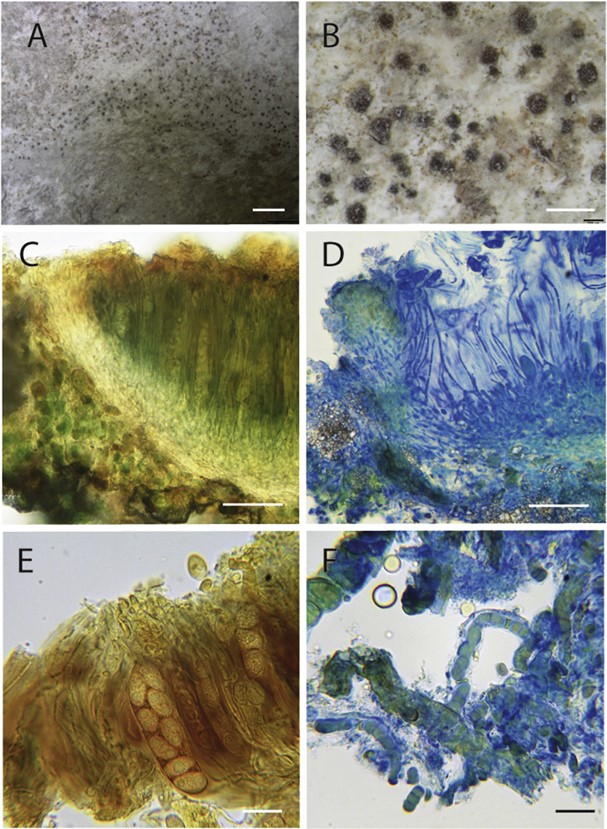
Fig. 3. Habit and morphology of Watsoniomyces obsoletus. A & B, Habit (from Powell 4724); C & D, sections through the hymenium and exciple, C in diluted Lugol’s, D in ink-vinegar; E, detail of ascospores in ascus, in Lugol’s without pretreatment with K; F, cyanobacterial chains and fungal hyphae from thallus (CeF from neotype). Scale bars: A ¼ 2 mm; B ¼ 0.5 mm; C & D ¼ 50 mm; E & F ¼ 20 mm.
