Volutella lini Mukerji, J.P. Tewari & J.N. Rai, Trans. Br. Mycol. Soc. 51(2): 337 (1968)
Index Fungorum number: IF 283327; MycoBank number: MB 283327; Facesoffungi number: FoF 10673; Fig. 1, 2
Saprobic on submerged decaying wood in a freshwater habitat. Sexual morph: Undetermined. Asexual morph: Coelomycetous. Colonies on the substrate effuse, scattered, whitish at first, becoming light yellow at maturity. Mycelium mostly immersed, composed of branched, septate, smooth, thin-walled, brown hyphae. Sporodochia with setae, 160–265 × 62–78 μm (x̅ = 198.6 × 69.7 μm, n =10) μm, sessile with slimy spore mass at the apex, swollen at the apex, hyaline, aseptate, subcylindrical to ovoid, with long acuminate setae arising from the base and margin, setae 95–255 μm long (x̅ = 157 μm, n = 30), 5.5 μm thick at the base tapering to 2 μm, erect, straight to curved, flexuous, unbranched, irregular in length, smooth, thick-walled, aseptate, cylindrical, tapering towards apex into an acute tip. Conidiophores aggregated into sporodochia, verticillate, with hyaline, thick setae around the side of conidiomata. Conidiogenous cells 8–12 × 1.5–3.0 μm (x̅ = 10.3 × 2.1 μm, n = 20), hyaline, subulate. Conidia 6–9 × 1–2.5 μm (x̅ = 7.5 × 1.7 μm, n = 50), produced at large numbers from the tips of the phialides, slimy, aseptate, hyaline, cylindrical or bacillar guttulate with rounded or acute ends, smooth, thin-walled, without mucilaginous sheath.
Culture characteristics – Ascospores germinating on malt extract agar (MEA) within 24 h. Germ tubes produced from the basal and apical cell of conidia. Colonies growing on MEA, reaching 25–30 mm in 4 weeks at 25 °C. Mycelia superficial, circular, with entire margin, flat, smooth, slimy, moist, from above light brown; reverse, light yellowish orange.
Material examined – Thailand, Tak Province, Tha Sing Yang, Ban Mae Ja Wang on decaying wood submerged in a freshwater river, 17 October 2019, O. Padaruth, CC63 (MFLU 22–0117), living culture, MFLUCC 22–0079.
GenBank accession numbers – LSU: OP216401, ITS: OP216406.
Known distribution (based on molecular data) – India (Cannon et al. 2012), Thailand (this study)
Known hosts (based on molecular data) – Unidentified host (Cannon et al. 2012, this study)
Notes – Volutella lini, introduced by Mukerji et al. (1968), was isolated from the rhizosphere and rhizoplane of Linum usitatissimum in India. The latest report of the species was by Cannon et al. (2012) from an unknown host in India and also provided the ITS sequence data of the two Volutella lini strains (CABI: IMI224502 and CABI: IMI92688). Our isolate, MFUCC 22–0079, is morphologically similar to other Volutella species and clustered with the strains of V. lini with 100% maximum likelihood bootstrap support and 1.00 Bayesian posterior probability (Fig. 63). Volutella lini MFLUCC 22–0079 has larger conidiomata (160–265 × 62–78 μm vs. up to 160 × 18.7–45.8 μm), longer setae (95–255 μm vs. 50–200 μm), and shorter conidia 6–9 × 1–2.5 μm vs. 8–14 × 1.2–1.6 μm) than the type strain, V. lini IMI 92688. The latter has a sporodochium with highly branched hyphae that become longer at mature stages and have sclerotia in the culture. The morphological differences observed may be due to the adaptation of V. lini to various environmental conditions in different habitats (Fig. 64). There are no differences in the ITS sequence data of the three strains of V. lini. Therefore, we introduce this isolate as a new geographical record of V. lini in Thailand and a new record also from freshwater habitat. Volutella lini is the second species of this genus reported from freshwater habitats, with V. citronella as the first one in Korea (Pangging et al. 2021).
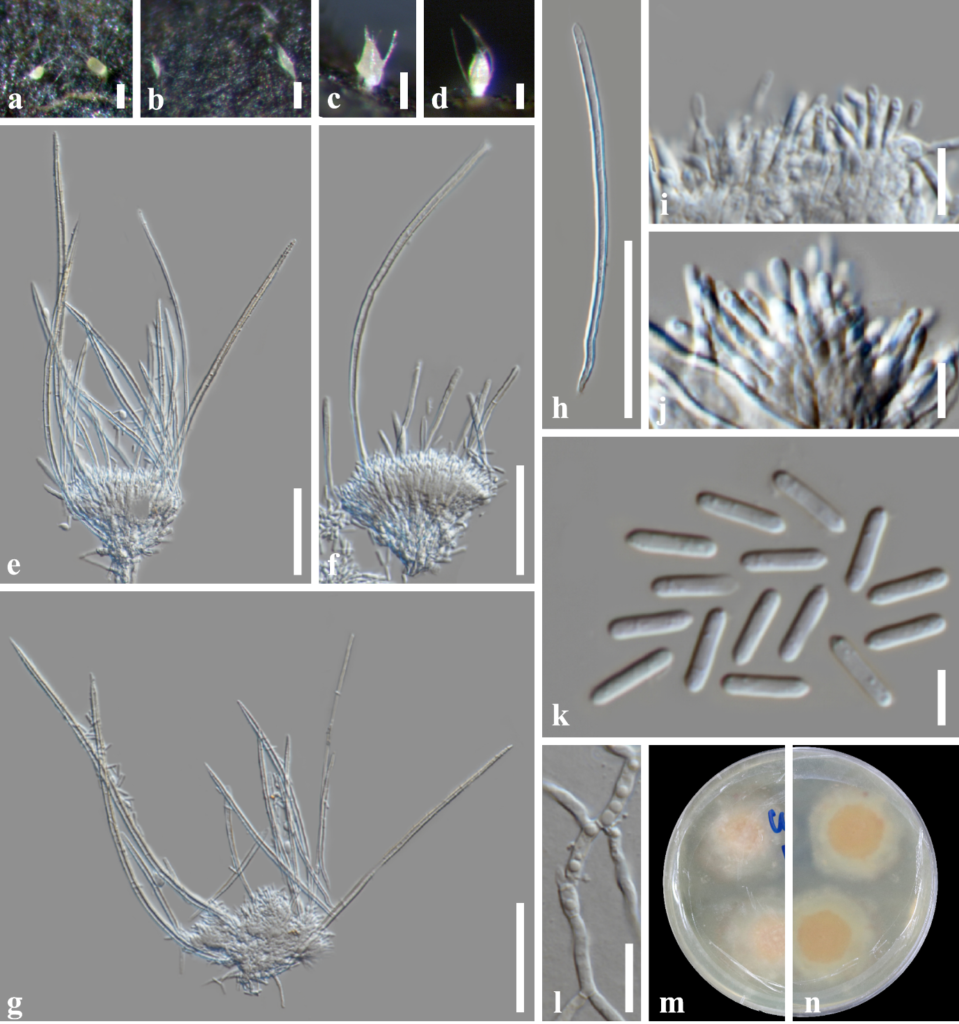
Fig. 1 – Volutella lini (MFLU 22–****, new geographical record and habitat record). a–d Appearance of sporodochial conidiomata on host. e–g Conidiophores, conidiogenous cells and conidium. h Setae. i, j Conidiogenous cells. k Conidia. l Germinated conidium. m, n Colonies on MEA from above and below. Scale bars: a–g = 50 μm, h–k = 5 μm, l = 20 μm.
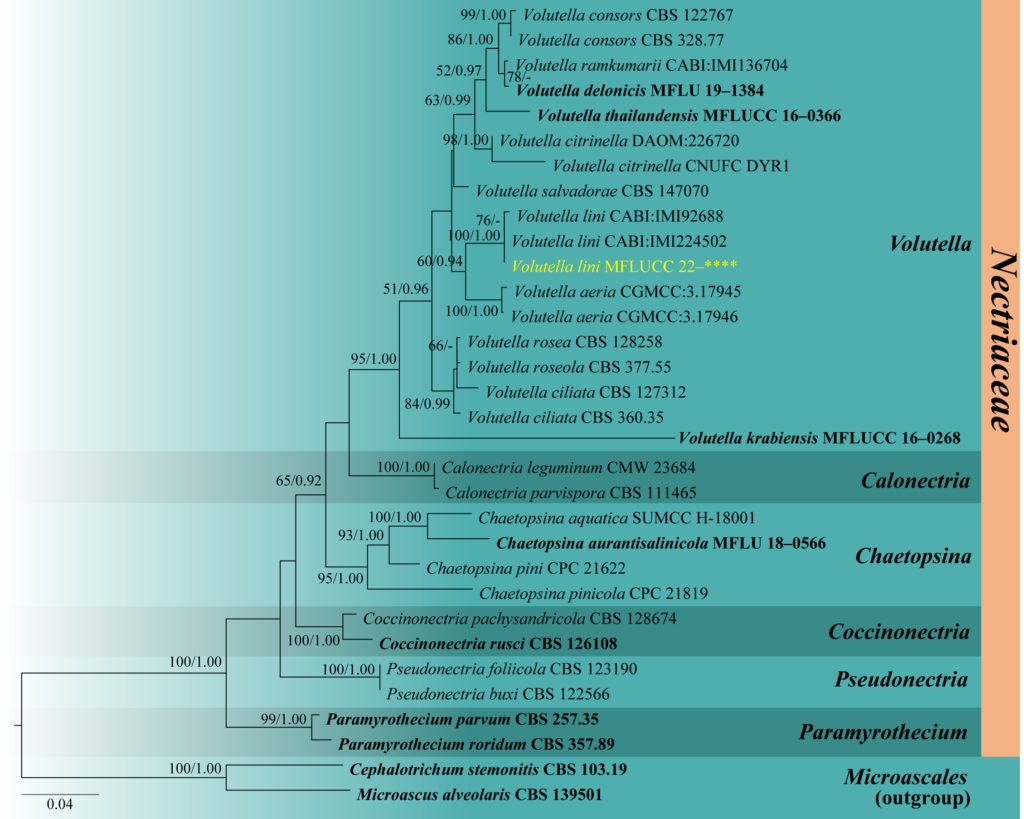
Fig. 2 – Phylogram generated from maximum likelihood analysis based on combined LSU and ITS sequence data. Thirty-two strains are included in the combined analyses which comprised 1391 characters (818 characters for LSU, 573 characters for ITS) after alignment. Tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best RaxML tree with a final likelihood value of –7336.851768 is presented. Estimated base frequencies were as follows: A = 0.242818, C = 0.245187, G = 0.283468, T = 0.228527; substitution rates AC = 1.514767, AG = 1.792179, AT = 2.073129, CG = 0.998833, CT = 4.606130, GT = 1.000000; gamma distribution shape parameter a = 0.229618. Bootstrap support values for ML greater than 50% and Bayesian posterior probabilities greater than 0.90 are given near nodes respectively. The tree is rooted with Cephalotrichum stemonitis (CBS 103.19) and Microascus alveolaris (CBS 139501). Ex-type strains are in bold and black. The newly generated sequences are indicated in yellow.
