Vinositunica Koh. Yamam., Degawa & A. Yamada, gen. nov.
Index Fungorum number: MB 829985; Facesoffungi number: FoF
Diagnosis: The purplish sporocarps and red-wine- colored chlamydospores differ from all other genera in Endogonales.
Etymology: Vinositunica (Latin), in reference to the characteristic wine-colored pigmentation on the peri- dium and outer wall of the chlamydospores.
Description: Sporocarp epigeous on soil surface or semihypogeous; reniform or irregular, short stipe-like sterile base often present; 2–20 mm wide. Peridium white and partially purple; single layer, sometimes indistinguishable from glebal tissue; composed of thick- or thin-walled aseptate hyphae. Gleba pale yellow or purplish-gray; composed of numerous chlamydos- pores and thick- or thin-walled aseptate hyphae. Chlamydospore radially or randomly distributed in sporocarp; terminal on single subtending hypha; broadly ellipsoidal; 50–700 μm diam; wall composed of brownish-purple or red-wine-colored outer layer and colorless inner layer; contents yellow, uniformly granular.
Type: Vinositunica radiata Koh. Yamam., Degawa & A. Yamada, sp. nov.
Notes: – Vinositunica is the only genus in Endogonaceae that forms chlamydospores but lacks an observation of sexual reproduction. Sphaerocreas and Densospora (Densosporaceae, Endogonales) resem- ble Vinositunica in the formation of chlamydospores and the lack of a sexual stage. However, these genera have colorless chlamydospore walls (McGee 1996; Yamamoto et al. 2015). Although Glomerales and Diversisporales in Glomeromycotina are also chlamy- dosporic and include sporocarpic species (Gerdemann and Trappe 1974; Yao et al. 1996; Błaszkowski 2012), no species in those taxa shows purplish-colored spor- ocarp tissue, dark red-wine-colored spore wall, and yellowish intracellular contents of spores, with two exceptions discussed below. The dark spore wall of Vinositunica suggests deposition of sporopollenin, which has been found in the spore walls of Glomeromycotina (Bianciotto and Bonfante 1999) and Mucorales (Gooday et al. 1973) and has the ability to defend against biological degradation and chemical stressors (Gooday et al. 1973; Bianciotto and Bonfante 1999).
At present, this genus includes only two newly described species from Japan. Although V. radiata consistently occurs under ectomycorrhizal trees, no mycorrhizae of Vinositunica were found and no plant mycobiont environmental sequences were included in the phylogenetic clade of this genus (FIGS. 1, 2). Therefore, it is necessary to determine whether Vinositunica forms ectomycorrhizal asso- ciations by field sampling and mycorrhizal synth- esis, as do several species of Endogone and Jimgerdemannia (e.g., Warcup 1990; Yamamoto et al. 2017b).
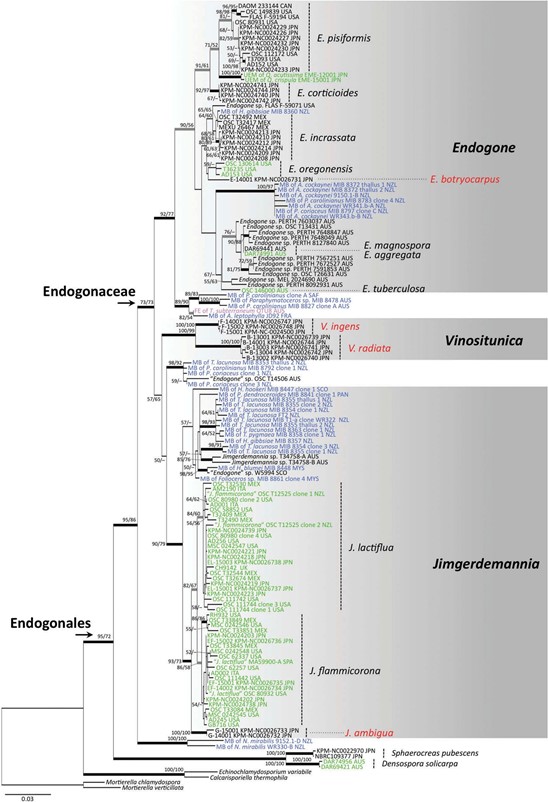
Figure 1. Maximum likelihood (ML) phylogenetic tree of the combined data set of 18S and 28S nuclear ribosomal DNA (nrDNA; data set 1). Phylogenetic relationships of four new species (red) of Endogonaceae and other species including plant mycobionts in Endogonales are shown. Mortierellaceae and Calcarisporiellaceae are used as outgroups. Bootstrap (BS) values (1000 replicates) >50% of ML (left) and maximum parsimony (MP) (right), respectively, are shown near the nodes. Branches supported by BS of ML/MP >70% (black) and ML >70% (gray) are highlighted with thick lines. Sequence ID is indicated by the voucher number and locality. Sequences shown in green = mycobionts of ectomycorrhizal root tips or sporocarps of putative ectomycorrhizal species; blue = mycobionts of bryophytes or ferns; pink = fine endophytes. Abbreviations: AUS= Australia; CAN = Canada; FRA= France; ITA = Italy; JPN = Japan; MEX = Mexico; MYS= Malaysia; NZL = New Zealand; PAN = Panama; SAF = South Africa; SCO = Scotland; SPA = Spain.
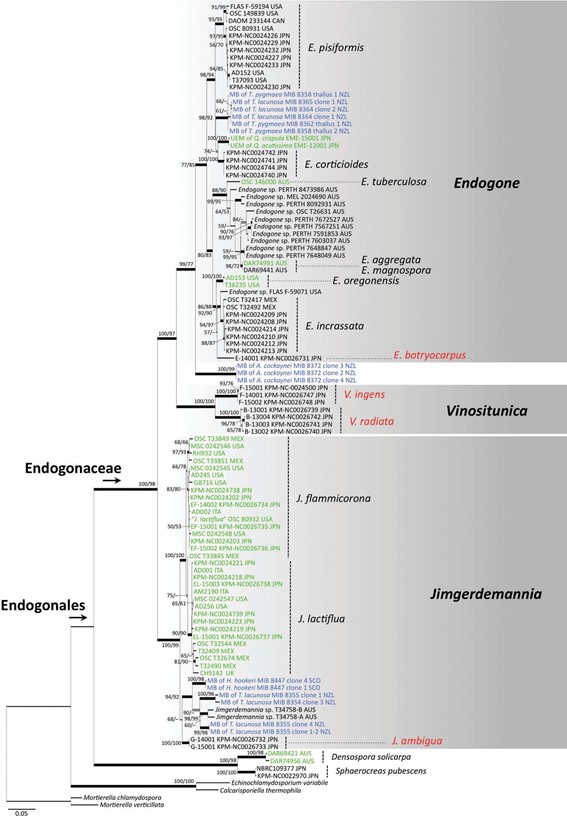
Figure 2. ML phylogenetic tree of the combined data set of the 28S nrDNA gene, tef1, and rpb1 (data set 2). Phylogenetic relationships of four new species (red) of Endogonaceae and other species including plant mycobionts in Endogonales are shown. Mortierellaceae and Calcarisporiellaceae are used as outgroups. BS values (1000 replicates) >50% of ML (left) and MP (right) are shown near the nodes. Branches supported by BS of ML/MP >70% (black) and ML or MP >70% (gray) are highlighted with thick lines. Sequence ID is indicated by the voucher number and locality. Sequences shown in green = mycobionts from ectomycorrhizal root tips or sporocarps of putative ectomycorrhizal species; blue = mycobionts of bryophytes. Abbreviations: AUS = Australia; CAN = Canada; ITA = Italy; JPN = Japan; MEX = Mexico; NZL = New Zealand; SCO = Scotland.
Species
