Pulverulina ulmicola (H.E. Bigelow) Matheny & K.W. Hughes, comb. nov.
MycoBank number: MB 835132; Index Fungorum number: IF 835132; Facesoffungi number: FoF; (Fig. 2A–E).
≡ Clitocybe ulmicola H.E. Bigelow, Beih. Nova Hedwigia 72:135. 1982 (basionym).
Pileus 6–35 mm wide, arched or plano-convex with slight depression at the center when young, becoming more depressed with age, margin decurved; surface dull, almost smooth in appearance but minutely silky under a hand lens; margin translucent striate when fresh, otherwise entire, not crenate; white or whitish (10YR 8/1) to very pale brown (10YR 7/3) or faintly grayish; very thin fleshed, not fragile, context white, odor absent, taste mild. Lamellae decurrent, (sub)distant with 16–20 L and several tiers of lamellulae, white to ivory, edges even, medium broad. Stipe 7–25 mm × 1–3 mm, curved, terete, dry, white, with a pruinose, pubescent, or fibrillose surface, point of attachment with some whitish mycelium, not insititious; tough, not fragile; context hollow or stuffed, white to whitish.
Basidiospores (6.5–)7–8.5(–9) µm × 4.5–5.5(–6) µm, mostly 7.5–8 µm × 5 µm, broadly ellipsoid to ovoid, smooth, thin-walled, inamyloid, with a small but discernible apiculus. Basidia 26–32 µm × 6–8 µm, 4–(2–) sterigmate. Pileipellis a cutis of cylindric hyphae 4–7 µm wide, end cells often protruding, with slightly thickened walls; context hyphae inflated, mostly 12–20 µm wide, thin-walled. Lamellar trama interwoven, hyphae 7–24 µm wide, hyaline, thin-walled or walls slightly thickened (–1.0 µm). Hymenial cystidia absent. Caulocystidia 30–82 µm × 6–8 µm, abundant, more or less cylindric or irregularly so with obtuse to subacute apices and forming a perpendicular turf with respect to stipe surface, at times without a basal septum and arising as 90-degree angled projections from stipe hyphae, thin-walled, hyaline; stipe hyphae parallel, thin-walled or with slightly thickened walls (–1.0 µm), hyaline. Clamp connections present.
Ecology and distribution: On bark of trunks of living Ulmus and Quercus, to date known only from the southern Appalachians, USA (Tennessee [type], North Carolina), July.
Specimens examined: USA. NORTH CAROLINA: Macon County, Highlands, Blue Valley, Forest Road 79, 35.0189°N, 83.2530°W, on bark of trunk of living Quercus, 17 Jul 2011, R.H. Petersen TFB13871 (TENN-F-065567) GenBank ITS = MT237476, 28S = MT237446. TENNESSEE: Blount County, Great Smoky Mountains National Park, Cades Cove, 35.6019°N, 83.8114°W, on bark of trunk of living Ulmus, 20 Jul 1966, L.R. Hesler LRH29208 (holotype TENN-F-029208, isotype NY 586623) GenBank ITS = HQ179667, 28S =HQ179668; Cumberland County I-40 rest area between exits 324–325, near Crab Orchard, 35.9092°N, 84.878°W, elev. 550 m, on bark of the trunk of a living white oak (Quercus), 21 Jul 2019, P.B. Matheny & J.C. Matheny PBM4303 (TENN-F-074865) GenBank ITS1 = MT239041, ITS2 = MT241732.
Notes: The description above is modified from Bigelow (1982) and includes observations based on recent material. Pulverulina ulmicola has the appearance of a small, whitish, marasmioid fungus, but the combination of the small size, distant decurrent lamellae, tough texture, interwoven gill trama, presence of long cylindri- cal caulocystidia, and short, ellipsoid, smooth basidiospores, and occurrence on bark of living oak trees match the protologue of Clitocybe ulmicola in all particu- lars. The structure of the pileipellis is not consistent with many marasmioid fungi. Furthermore, molecular annotation of the holotype collection enabled a sufficient genotype match using ITS and 28S DNA sequence data. All 3 collections fruited between 17 and 21 July, so the phenology of this species suggests a narrow window of time of fruiting, which could explain why it is rarely collected.
In systematic studies of Porotheleaceae, both Antonín et al. (2019) and Vizzini et al. (2019) depict Pulverulina ulmicola (as Clitocybe ulmicola) in phylogenetic tree figures labeled together with Porotheleum fimbriatum as “Porotheleum”. The genus Porotheleum (homotypic synonym Stromatoscypha Donk) is typified by P. fimbria- tum, a reduced cyphelloid fungus that produces “aggregates of fruiting bodies that are densely crowded on a membranous subiculum” (Bodensteiner et al. 2004 and references therein). Because Porotheleum is more closely related to other agarics and to several poorly identified environmental isolates (Fig. 1), and because of differences in basidiome morphology, we have accommodated Clitocybe ulmicola in a new separate genus, Pulverulina.
Hydropus sensu Singer (1986) is polyphyletic. The genus in its strict sense is probably restricted to species with amyloid spores, that lack pleurocystidia, and that produce latex (Kühner 1938, Moncalvo et al. 2002). Pulverulina has inamyloid spores and does not produce latex. The sister lineage of P. ulmicola is “Hydropus” sp. RV98/43, but this is not a Hydropus in the strict sense (Fig. 1). We have not examined the material (Moncalvo et al. 2002) from which only the 28S sequence was analyzed, but morphologically it may be more similar to Pulverulina.
Per He et al. (2019), species of Trogia have a habit (clitocyboid to omphalinoid) and a tough texture similar to Pulverulina but have the ability to revive. We have no data that suggests Pulverulina is marcescent, but the basidiomes are tough in texture. Trogia, however, is polyphyletic in our analysis, and all are distantly related to Pulverulina. Of these, Trogia aff. furcata (Desjardin and Perry 2017) is most closely related to Pulverulina but differs from it by forked lamellae or venose- reticulate hymenophore, darker basidiome colors, and phylogenetic position closer to Porotheleum (Fig. 1). Trogia aff. furcata possesses cheilocystidia, lacks the dense caulocystidiate surface of Pulverulina, and has sarcodimitic lamellar trama. The la- mellar trama of Pulverulina is composed of interwoven, thin-walled, often-inflated hyphae, which occasionally do have thickened walls—up to 1.0 µm thick.
Collections to which we attributed the name Hydropus omphaliiformis (TENN- F-074844; = Marasmiellus omphaliiformis Kühner (Desjardin 1997)) could be confused with Pulverulina ulmicola in the field because the former also occurs on bark of trunks of living oak trees and produces small clitocyboid basidiomata, but the pubescent stipe is darker in color (olive-buff to dark brown) than that of Pulverulina ulmicola, and the stipe is longer than the pileus diameter. It shares superficial simi- larities to Trogia aff. furcata (Desjardin and Perry 2017), and both occur in the same clade together with Porotheleum. Hydropus omphaliiformis was originally described in Marasmius Fr. and then transferred to Marasmiellus Murrill and then Hydropus. None of these generic assignments are satisfactory based on phylogenetic results, assuming our material is reliably identified. This portion of Porotheleaceae, as well as other lineages in the family, requires more attention and eventual systematic revision. For example, the genus Inflatostereum D.A. Reid may belong in this family based on 91–93% sequence similarity of Inflatostereum aff. glabrum (AB971705) to samples of Gerronema. Note that the Porotheleaceae as recognized by Vizzini et al. (2019) is reduced compared to the concept of the family here in a broad sense (Fig. 1). The concept of Porotheleaceae sensu He et al. (2019) contains only 2 genera (Porotheleum, Phloeomana), which is not a monophyletic group.
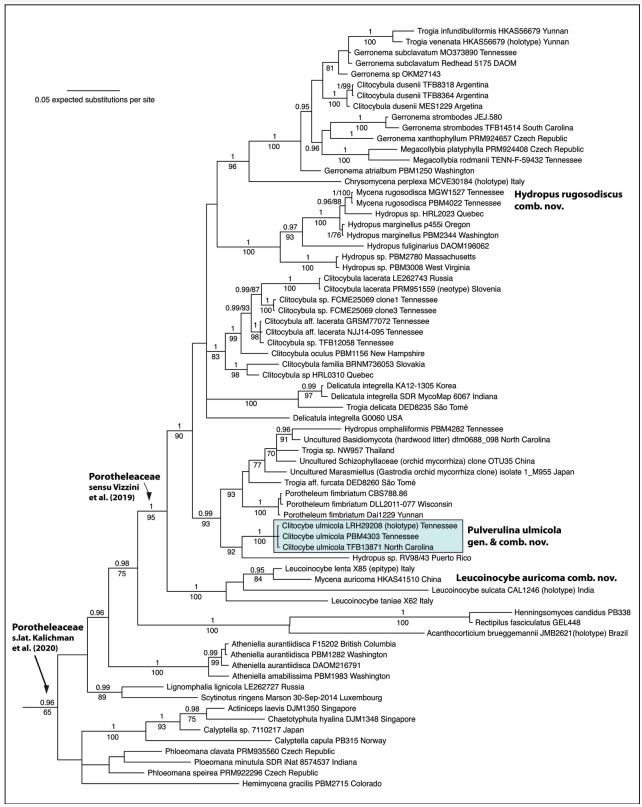
Figure 1. BI phylogeny of the Porotheleaceae based on a supermatrix of ITS and 28S nucleotide sequences. Baeospora and Cheimonophyllum (Cyphellaceae) were used for root- ing purposes but pruned from the tree figure. BPPs ≥0.95 and ML bootstrap values >70% are shown. Porotheleaceae sensu Vizzini et al. (2019) is the same as Porotheleaceae s.str. of Kalichman et al. (in press). Porotheleaceae s.lat (Kalichman et al., in press) equals reference to the “hydropoid clade” (Matheny et al. 2006, Moncalvo et al. 2002). The branch leading to Acanthocorticium, Rectipilus, and Henningsomyces was artificially shortened for better graphical presentation.
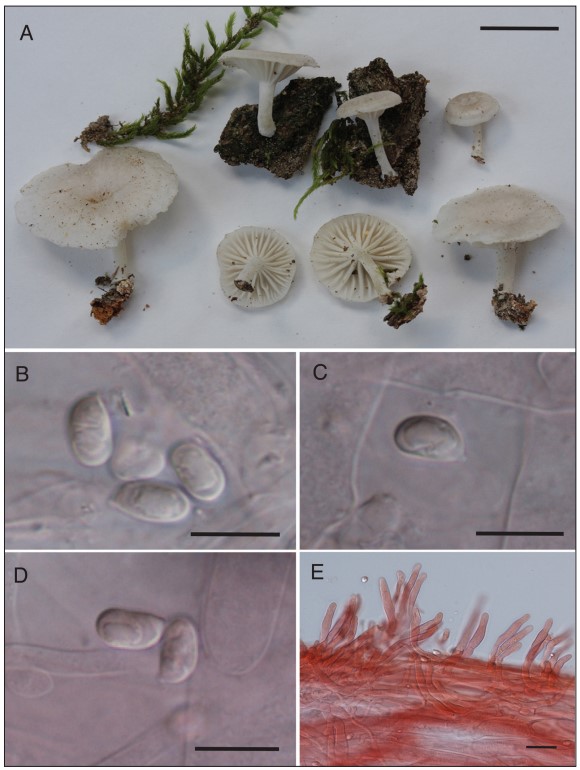
Figure 2. Pulverulina ulmicola (PBM4303). A. Basidiomata. Scale bar = 10 mm. B–D. Ba- sidiospores from pileipellis tissue. Scale bars = 10 µm. E. Caulocystidia. Scale bar = 25 µm.
