Paramycetinis austrobrevipes R.H. Petersen, sp. nov. Figs 3–8
MycoBank number: MB 555793; Index Fungorum number: IF 555793; Facesoffungi number: FoF;
Differs from Paramycetinis caulocystidiatus by its broader rhizomorphs that form loose nets, by its basidiomata that arise as rhizomorph branches and/or from woody substrate, and by its broom cell-like cheilocystidia.
Type: Australia, Tasmania, Gordon-Pedder National Heritage Area, Rainforest Nature Walk vicinity, 8.VI.1991, coll. RHP & KWH, TFB 4033 (Holotype TENN-F-050135).
Etymology: referring to micromorphological similarities to Gymnopus neobrevipes.
Basidiomata (Fig. 3) marasmioid, diminutive, arising as branches from rhizomorphs and/or directly from woody substrate. Pileus 2–12 mm broad, convex to shallowly conical, sometimes abruptly or broadly umbonate and even then abruptly umbilicate, deeply sulcate-striate to non-striate, matte, at high magnification (50×) telltale wispy white hairs can be seen in the sulcate depressions; disc (including umbo) 5F6 (“Prout’s brown”), 6E3 (“hair brown”), 9D3 (“benzo brown”), 7C4 (“wood brown”), 6E4 (“fuscous”), 7C6 (“Mikado brown”) to 9C3 (“cinnamon drab”); limb 6C5 (“sayal brown”), 6B5 (“cinnamon”), 17B2 (“light drab”), 7B2 (“tilleul buff ”), to 9B2 (“vinaceous buff ”), 8D5 (“army brown”), 9C3 (“cinnamon drab”), 9B3 (“light cinnamon drab”) to 7C5 (“fawn color”); margin 6A3 (“pinkish buff ”) to 4A3 (“cartridge buff ”). Lamellae adnexed, subdistant (total lamellae 20–24), about 1 mm broad, thickish, without anastomoses, 7B2 (“tilleul buff ”), 9B2 (“vinaceous buff ”), 6B4 (“cinnamon buff ”) to 6A3 (“pinkish buff ”), not marginate; dried lamellar trama dark brown and glassy, as though gelatinized. Stipe 18–85 × 0.8–1.2 mm, terete, stuffed (not hollow), appearing glabrous–shining but minutely pruinose at least upward and downward (35×), black to abruptly 6C5 (“sayal brown”), 6B3 (“cinnamon buff ”) or mahogany at very apex (not concolorous with lamellae), downward totally black; medullary portion cloud gray, cortical layer thin, nearly black (40×); insertion non-insititious; vesture sparse, pallid, near 7B2 (“tilleul buff ”). Rhizomorphs >1 mm broad, gradually tapering to 0.2–0.5 mm distally, terete or somewhat compressed, extensive to almost absent, often forming a rudimentary net. Odor negligible; taste negligible or weak of garlic; consistency tough then mealy.
Habitat & phenology: Nothofagus wood (and then densely gregarious) and twigs, at or near forest floor, Arthrotaxis dead branchlets; to this time, May–June.
Pileipellis constructed of the following: 1) slender hyphae 2–3.5 µm diam, thick-walled (wall <0.5 µm thick), sparsely encrusted (Fig. 4A) with ornamentation appearing as flakes adhered to outer hyphae wall; 2) slender hyphae 1.5–3 µm diam, firm-walled with slender, sometimes awl-shaped diverticula (Fig. 4B, 5A–D), obscurely clamped, forming a indiscrete layer in a mucoid matrix (i.e. significant debris including bacteria and collapsed spores, Phc); 3) slender, coralloid hyphal termini (Fig. 5e,F); 4) thicker hyphae 4–12 µm diam, refringent (Phc), infrequently branched, meandering to appear a bit like lattice, thick or gelatinized–walled (wall <2.5 µm thick), with outer profile smooth and clear but inner wall contour irregular, obscurely clamped; termini occasionally more or less erect as “pileal hairs.” Pleurocystidia (Figs 6B, 7A–c) common, 26–33 × 5–9 µm, fusiform to mucronate, conspicuously clamped, usually with partitioned contents. Basidia 23–28 × 6–9 µm, clavate, 4-sterigmate (sterigmata <6 µm long, somewhat bowed, stout), obscurely clamped; contents homogeneous. Basidiospores (Fig. 6A) 6–9 × 3.5–4.5 (–5) µm (Q = 1.502.43; Qm = 1.92; Lm = 7.47 µm), more or less pip-shaped but often tapered slightly proximally (that is, perhaps marasmioid), smooth, thin-walled, inamyloid; contents homogeneous. Cheilocystidia (Figs 6D, 7e–H) locally common to scattered among basidia, 17–32(–40) × 5–12 µm, clavate to ventricose-rostrate, obscurely clamped, thin-walled below, firm- walled above, surmounted by a corona of extremely complex, vermiform to slender-digitate, refringent (Phc) diverticula; diverticula <7 × 0.71 µm, gnarled, often branched. Stipe Medullary hyphae hyaline, 2–5.5 µm diam, firm- to thick-walled, seldom but conspicuously clamped;cortical hyphae adherent, 3–7 µm diam, yellow singly, dark ochraceous brown and refringent in mass (Phc), involved in a thin film of mucus, with side branches ranging from simple lobes to caulocystidia. Caulocystidia (Fig. 8) scattered, <105 × 46 µm (at widest point, narrowing to 3–3.5 µm diam at base, 2–3 µm diam at apex), gregarious but more or less evenly distributed, usually single, rarely in pairs, as side branches of stipe surface hyphae, apparently near the terminus of such hyphae (and therefore appearing as though from an asymmetrical origin, yellow and refringent (Phc), so refringent that wall thickness is hardly discernible (when wall seems to be discernible, 1–1.5 µm thick), narrowly rounded apically and apparently without adherent debris, dextrinoid (Melzer’s reagent + Phc) or brown (Melzer’s reagent + BF), not internally septate.
Commentary: Pileus shape in Pa. austrobrevipes varies from convex with an abrupt, distinct umbo to a gradual and shallow umbo to no evidence of an umbo. In all cases, the disc (with or without umbo) is significantly darker than the limb.
The pileus micromorphology of Pa. austrobrevipes approaches that of the North American Marasmius brevipes [ Gymnopus neobrevipes R.H. Petersen &
Hughes 2019)]. Basidiomata of both species arise on woody substrate (usually twigs with thin bark intact or occasionally as branches from an extensive rhizomorph net), but G. neobrevipes basidiomata are small and thumb tack- shaped while those of Pa. austrobrevipes are tall and mycenoid. Pileus colors are similar but also characteristic of several taxa in Gymnopus sect. Androsacei. Rhizomorphs and stipes are black, although stipe in G. neobrevipes is glabrous throughout its length. There is no record of a weak or latent taste of garlic for G. neobrevipes. Comparison of notes and drawings of G. neobrevipes specimens indicate that it also has a gelatinizing pileipellis, but with a more demonstrable “ramealis-structure.” Gymnopus neobrevipes basidiomata also occur independently from rhizomorphs as well as branches from rhizomorphs, and its rhizomorphs are also thick and form an aerial network as well as on the substrate surface. Despite these similarities, molecular evidence shows Pa. austrobrevipes and G. neobrevipes to be only distantly related (see Fig. 1).
The surviving culture of TENN-F-053181 (TFB 3585) formed considerable aerial mycelium and what appears to be resupinate rhizomorphs against the side of the MEA-slanted storage test tube. This is different from other cultures of the same putative species but identical to a culture of TFB 3966, of which voucher basidiomata are no longer extant.
Collections of Pa. austrobrevipes may be mistaken in the field for M. crinis-equi. The latter can be distinguished in the field by collariate lamellae, significantly smaller basidiomata with short, curved stipes and typical hymeniform pileipellis and cheilocystidia. Grgurinovic (1997) cited collections of M. crinis-equi from New South Wales and South Australia, and Pegler (1977) furnished a much wider range for the species.
Close inspection of rhizomorph surfaces shows that when rhizomorphs branch, whether to produce a basidiome or a sterile branch, there is often a rupture of the parent rhizomorph cortex—splitting the cortex and revealing the medulla. This rupture is sparsely covered by a brown mycelium, which also covers the basal portion (<50 µm) of the new branch. Whether sterile or fertile, the new branch is also vestured with caulocystidia, which peter out in less than a millimeter distally, leaving the greater portion of the rhizomorph branch (or stipe) subglabrous.
On whole wheat flour agar (Farnett & al. 1999), cultures of Pa. austrobrevipes produced chiefly submerged mycelium, no textura intricata, and no rhizomorphs. TFB 3591 (TENN-F-053146) produced a minutely granular white mycelial mat with ill-defined margins.
Text and illustrations of Paramycetinis species were generously pre-reviewed by Australian and New Zealand mycologists in order to avoid nomenclatural duplication of any taxon already described. While no present-day match was suggested (but see below), five historical names under Marasmius were pointed out, as follows:
Marasmius eucalypti Berk., in Hooker, Fl. Tasman. 2: 249, 1859 [“1860”].
Pegler (1965: 331) examined the type specimen. While a basidioma is illustrated as arising as a side branch of a rhizomorph, pileipellis was described as “hymeniform, composed of diverticulate elements similar to the cheilocystidia.” Thick-walled caulocystidia are described and illustrated. All of this indicates identification as a Marasmius, probably M. sect. Sicci.
Marasmius meloniformis Berk., in Hooker, Fl. Tasman. 2: 249, 1859 [“1860”].
Pegler’s (1965: 339) study of the type specimen described surviving material in poor condition. The “long, setaceous stipes, which arise as branches from
creeping rhizomorphs” must resemble the habit of Paramycetinis austrobrevipes. Conversely, the following characters do not match: 1) pileus 1–2 mm broad, strongly plicate; 2) lamellae few; 3) epicutis hymeniform; 4) cheilocystidia broom cell-like. Pegler (1965) concluded: “The species clearly belongs in the genus Marasmius, sect. Marasmius.”
Marasmius subsupinus Berk., in Hooker, Fl. Tasman. 2: 249, 1859 [“1860”].
From Berkeley’s description the following is inferred: Basidiomata apparently conchate with a very short, curved stipe. Lamellae “so thick and rigid that this pretty species might almost be placed in Lentinus.” “It varies in color from nearly white to rufous.”
Marasmius emergens Cooke, Handb. Austral. Fungi: 88, 1892.
Protologue: “Very minute, white, suberumpent; pileus convex (1 mm broad); stem abbreviated, or sometimes elongated, curved, ascending; gills distant, few, white. On wood. Tasmania.”
Marasmius subroseus Cooke & Massee, Grevillea 21: 37, 1892.
The protologue implies the following: basidiomata caespitose; no mention of rhizomorphs; “stem becoming a little reddish downwards and clad at the base with white pubescence;” gills distant. Unless proven otherwise, the organism can be accepted as a Marasmius.
In addition to these names, an undescribed Australian species is well-known under the informal name of Marasmius “angina.” Online, numerous photos and one description (http://www.elfram.com/fungi/fungi_l/marspang_a.html; https://www.bushheritage.org.au/blog/fungi-and-citizen-science-in-the-liffey- valley) show an unmistakable resemblance to Paramycetinis austrobrevipes, except that the basidiomata are distinctly larger and no rhizomorphs appear in any photo. There is no reason (yet) to consider Marasmius “angina” as anything but a true Marasmius.
Additional specimens examined: AUSTRALIA, Tasmania, Cradle Mountain National Park, Waldheim Nature Track, 31.V.1991, coll. RHP & KWH, TFB 3977 (TENN-F-053232); Geeveston, Tahune Forest Preserve, 5.VI.1991, coll. RHP & KWH, TFB 4008(TENN-F-050195);Gordon-Pedder National Heritage Area, Rainforest Nature walk vicinity, 8.VI.1991, coll. RHP & KWH, TFB 4038 (TENN-F-050212); Hobart, Mt. Wellington, gully at 400 m elev., 25.V.1991, coll. KWH, TFB 3591 (TENN-F-053146); coll. RHP & KWH, TFB 3585 (TENN-F-053181); Lake Pedder National Heritage Area, 26.V.1991, coll. RHP & KWH, TFB 3917 (TENN-F-050119); Lake St. Clair National Park, Mt. Rufous track, 29.V.1991, coll. RHP & KWH, TFB 3945 (TENN-F-050073); 8-10 kms north of Rosebery, slopes of Mt. Murchison, 30.V.1991, coll. RHP & KWH, TFB 3949 (TENN-F-051333); coll. RHP, TFB 3966 (TENN-F-053231).
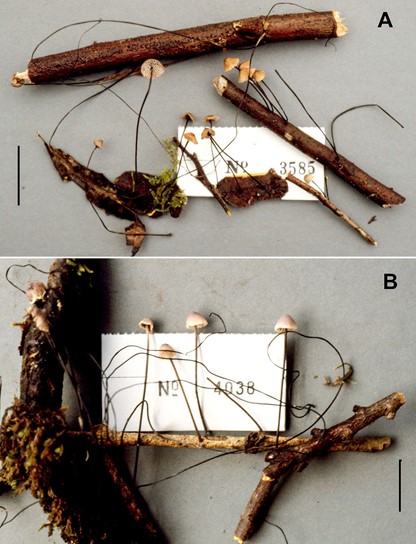
Fig. 3. Paramycetinis austrobrevipes. Habit. A. (TENN 53181). B. (TENN-F- 050212). Scale bars: 20 mm.
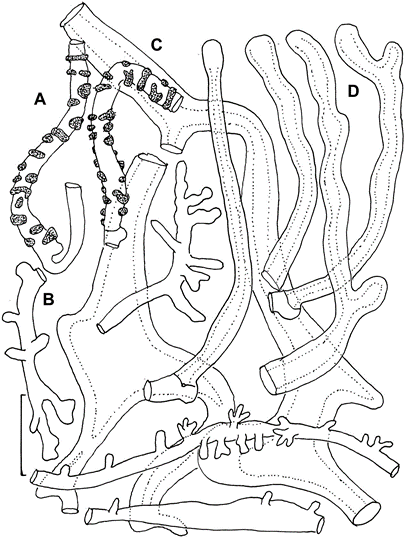
Fig. 4. Paramycetinis austrobrevipes. Structures of pileipellis. A. Encrusted hyphae (TENN 50119). B. Diverticulate hyphae (TENN-F-050135). C. Thick-walled lattice hyphae (TENN-F-050135). D. Pileal hairs (TENN-F-050119. Scale bars: 20 µm.
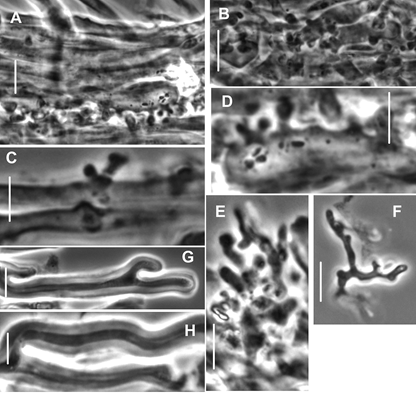
Fig. 5. Paramycetinis austrobrevipes (TENN-F-050135). Pileipellis elements. A. Layer of diverticulate hyphae as embedded within pileipellis. B. Layer of diverticulate hyphae. C,D. Individual diverticulate hyphae. E, F. Broom cell-like hyphal termini. G,H. Hyphae with gelatinized walls. Scale bars: 10 µm.
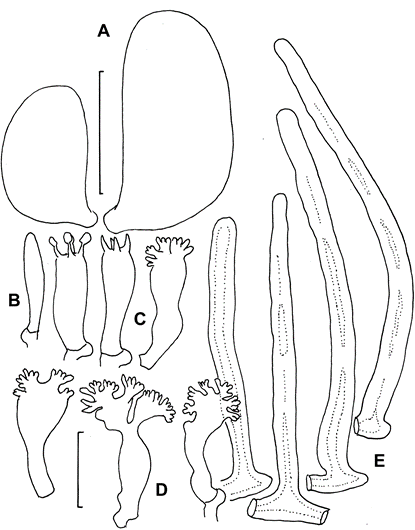
Fig. 6. Paramycetinis austrobrevipes (TENN-F-050135). A. Basidiospores. B. Pleurocystidium. C. Basidia. Cheilocystidia. E. Caulocystidia. Scale bars: A = 5 µm; B–D = 20 µm.
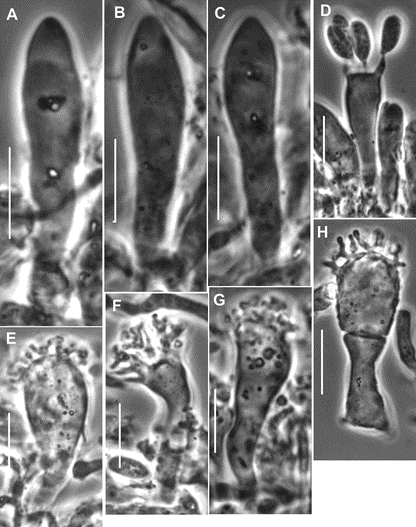
Fig. 7. Paramycetinis austrobrevipes (TENN-F-050135). A–C. Pleurocystidia. D. Basidium. E–H. Cheilocystidia. Scale bars: 10 µm.
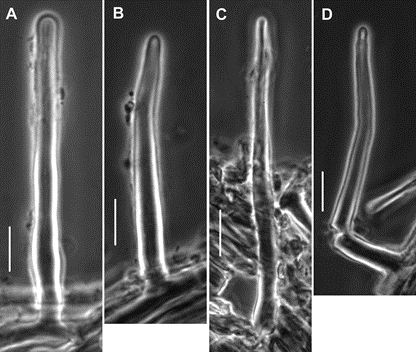
Fig. 8. Paramycetinis austrobrevipes (TENN-F-050135). Caulocystidia. Scale bars: 10 µm.
