Hormonema carpetanum Bills, Peláez & Ruibal, in Bills, Collado, Ruibal, Peláez & Platas, Stud. Mycol. 50(1): 152 (2004)
Index Foungorum Number: IF 500039; Facesoffungi Number: FoF 15671
= Hormonema sp., Peláez et al., Syst. & Appl. Microbiol. 23: 333. 2000.
Synanamorph – Sclerophoma sp.
Etymology – Carpetanus (Latin), in reference to the ancient Roman name for the Central Cordillera of Spain
Mycelium consisting of torulous, wide, often thick-walled, hyaline to dark olive to olive black, short-cylindrical to subglobose cells, often with hyphal cells longitudinally septate, in age sometimes developing groups of muriform cells, with individual cells up to 22 µm diam, generally sparsely branched, but sometimes becoming more branched with age. Conidiophores absent on mycelia. Conidiogenous cells holoblastic, integrated, intercalary, usually not differentiated from main axes of vegetative mycelium, occasionally arising from an undifferentiated lateral cell or filament, sometimes with a faint pore or slightly protruding annellate scar evident at the conidiogenous locus, often abundant along the entire radius of young extending hyphae, less so on older, contorted and melanized hyphae. Conidia up to 7–14(–16) µm long, up to 3–6 µm wide, aseptate, or rarely 1-septate in old cultures, usually ellipsoidal and tapered towards the base, but quite variable, occasionally pyriform or subglobose, smooth, often with one or more budding scars, hyaline to pale olivaceous-grey, often budding to produce 1 or 2 secondary conidia, accumulating in yeast-like masses along radial axes of vegetative hyphae. Pycnidial conidiomata in agar or on sterilized leaves up to 350 µm diam, globose to subglobose, unilocular, solitary to gregarious, rarely confluent in age, shiny, dark olive-brown to black, smooth, or with scanty 30–75 µm long, basally up to 7 µm wide setae; conidiomata initiating as stromatic masses of broadly cylindrical to isodiametric cells, enveloped by a thin layer of hyphal filaments, dehiscing by irregular erosion of upper layers, accumulating dark brown masses of moist conidia when mature. As basal stromatic cells of conidiomata accumulate and expand, upper layers of stromatic cells convert into conidiogenous cells. Conidiogenous cells phialidic, determinate, short cylindrical to isodiametric, 8–15 µm diam, thin- to thick-walled, hyaline to pale, giving rise to one or more conidia through one or more poorly defined openings or by conversion of the cell’s internal contents directly into a conidium, collapsing after conidia are released. Pycnidial conidia ellipsoid to broadly ellipsoid, sometimes curved, indented on one side or constricted at the centre, smooth, usually tapered toward the base, occasionally with the eccentric basal scar, hyaline to dark greyish brown, 8–14(–17) × 4–6 µm.
Cultural characteristics – Colonies on PDA differed in radial extension depending on the isolate, ranging from 21–37 mm diam, with the margin submerged, fimbriate, usually radially rugulose or slightly furrowed, often slightly depressed toward the centre, consisting predominantly of submerged, radially extending hyphae, shiny to moist, during the first few weeks, aerial mycelium absent to scant but abundant aerial filamentous mycelium may emerge with pro-longed incubation (>1 mo) in all strains scattered to gregarious pycnidia formed in about 3–4 weeks at or below the agar surface, usually with masses of moist conidia from mycelial conidiogenesis accumulating in moist drops or in radial strands, pale olive-brown to dark olive, Yellowish Olive, Dark Olive, Dark Greenish Olive, 3F6–2, 4F6–2, or dark olive-grey, Dark Greyish Olive, Olivaceous-Black (1), 30F5–2, eventually becoming black. Colonies on OM ranged from 24–32 mm diam; depending on the isolate, submerged to appressed, radially furrowed, silky to shiny, moist, dark olive, olivaceous-black to black, in most strains scattered pycnidia formed at or below the agar surface in about 3–4 wks. Colonies on CYA 17–21 mm, raised, rugulose, with radially extending yeast-like hyphae, often with hyaline to buff watery zones or sectors mixed with dark olivaceous-black zones. Growth was poor on media with dilute starch as the primary carbon source, e.g., cornmeal agar or potato-carrot agar. No growth was observed at 37 °C.
Habitat – Isolated from surface-sterilized leaves of Juniperus species, plant litter, and rock surfaces.
Known distribution – Spain, from Burgos southward to Granada and from Ávila eastward to Guadalajara.
Specimens examined – Spain, Madrid, La Cabrera, Cancho Gordo, isolated from surface of granite formation, Aug. 2001, dried holotype culture with mycelial conidia and pycnidia conidiomata and ex-holotype culture TRN25 = CBS 115712.
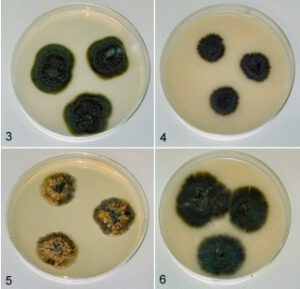
Figure 1 – Hormonema carpetanum in agar culture after two weeks. 3. TRN40, on potato-dextrose agar. 4. TRN40, on oatmeal agar. 5. F059461, on Czapek yeast extract agar. 6. F059461, on oatmeal agar.
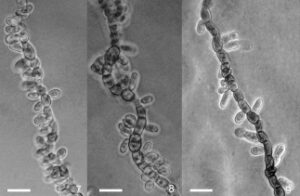
Figure 2 – Mycelial conidiogenesis and conidia of Hormonema carpetanum. 7, 8. F131395, slide culture on dilute malt agar. 9. TRN 31, slide culture on dilute malt agar. Scale bars = 10 µm.

Figure 3 – Pycnidia of Hormonema carpetanum. 10. ATCC 74360, pycnidia on autoclaved leaves of Juniperus oxycedrus. Scale bar = 500 µm. 11. TRN31, pycnidia on autoclaved leaves of J. oxycedrus. Scale bar = 500 µm. 12. TRN201, pycnidia from edge of oatmeal agar plate. Scale bar = 500 µm. 13. TRN201, pycnidia from edge of oatmeal agar plate. 14. Scale bar = 250 µm. TRN24, pycnidial initial from potato dextrose agar. Scale bar = 50 µm.
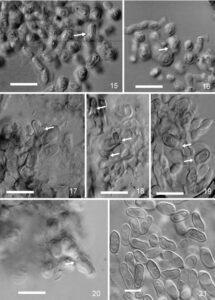
Figure 4 – Pycnidial conidiogenous cells and conidia of Hormonema carpetanum. 15. F131395, young stromatic cells and conidiogenous cell (arrow). 16. F131395, young stromatic cells and conidiogenous cell (arrow). 17. TRN201, stromatic cells and conidiogenous cell (arrow), note collapsed stromatic cells. 18. TRN201, stromatic cells and conidiogenous cells (arrows), note collapsed stromatic cells. 19. TRN201, stromatic cells and conidiogenous cells (arrows). 20. F131395, conidiogenous cell (arrow). 21. TRN31, pycnidial conidia produced on autoclaved leaves of J. oxycedrus. Scale bars = 10 µm.
