Codinaeella minuta (Tubaki) Réblová & Hern.-Restr., comb. nov.
MycoBank number: MB 842207; Index Fungorum number: IF 842207; Facesoffungi number: FoF; (Figures 27 and 28).
Basionym. Menispora minuta Tubaki, J. Hattori bot. Lab. 20: 166. 1958.
Description on the natural substrate: Colonies effuse, grey-brown to olivaceous grey, composed of setae and conidiophores, mycelium semi-immersed or immersed. Anamorph. Setae 123–184 µm long, 3–4.5 µm wide near the base, crowded, grow singly or in groups of two, erect, straight or flexuous, septate, smooth, dark brown, paler and thinner-walled toward the apex, unbranched, apex pale brown to subhyaline with a terminal or 1–3 lateral phialidic openings. Conidiophores 32–108 µm long, 3–4 µm wide near the base, macrone- matous, crowded, arise singly or in groups of 2–3, scattered among the setae, sometimes aggregate in loose columns, unbranched, erect, straight or flexuous, septate, smooth, pale brown, subhyaline toward the apex, sometimes reduced to single conidiogenous cells. Conidiogenous cells 20.5–35 × 2.5–3.5 µm tapering to 1.5–2 µm below the collarette, in- tegrated, terminal, mono- and polyphialidic with 1–5 lateral openings while internally septa can be formed, extending percurrently and sympodially, cylindrical, pale brown, sub- hyaline toward the apex, usually bearing persistent remnants of the collarettes; collarettes 3–4 µm wide, ca. 2 µm deep, funnel-shaped, subhyaline, the apical part soon evanescent. Conidia 13–18 × 2.5–3.5 µm (mean ± SD = 16.2 ± 1.1 × 2.8 ± 0.3 µm), falcate, tapering toward both ends, slightly truncate at the base with an inconspicuous scar, aseptate, hya- line, with a straight or gently curved setula at each end, 5.5–8.5(–10) µm long, inserted terminally at the apex, subterminally at the base, conidia accumulate in slimy whitish fascicles. Teleomorph. Unknown.
Culture characteristics: On CMD: colonies 61–65 mm diam, circular, flat, margin entire, lanose, floccose, cobwebby toward the margin, becoming mucoid, varying in color due to the absence of aerial mycelium, beige brown to pale banana yellow, occasionally orange with a yellow-gold margin, pale ochre to orange pigment diffusing into agar, reverse beige, ochre-beige to orange. On MLA: colonies 42–45 mm diam, circular, flat, margin entire to fimbriate, lanose, floccose becoming mucoid, grey-brown to dark brown when aerial mycelium is present, deep carrot orange to dull orange brown with orange-gold margin when mucoid, pale orange pigment diffusing into agar, reverse orange to dark amber. On OA: colonies 58–63 mm diam, circular, raised, margin entire, sparsely lanose becoming mucoid, beige-grey to dark grey toward the margin when aerial mycelium is present, orange with tawny margin when mucoid, sometimes orange to ochre pigment diffusing into agar, reverse dark grey-brown or amber. On PCA: colonies 70–73 mm diam, circular, flat, margin entire to fimbriate, sparsely lanose becoming mucoid, grey-brown, tawny or orange with olivaceous brown margin on mucoid spots, pale orange pigment diffusing into agar, reverse dark olivaceous brown to amber. Sporulation was abundant on all media.
Description on CMA with U. dioica stems. Colonies effuse, mycelium composed of branched, septate, hyaline to subhyaline hyphae 1.5–3 µm diam. Anamorph. Setae absent, conidiophores, conidiogenous cells, and conidia similar to those from nature. Conidio- phores 46–297(–450) µm long, 3–3.5 µm wide near the base, branched or unbranched, erect, septate, smooth, pale to medium brown. Conidiogenous cells 13.5–32.5 × 3.5–4.5 µm tapering to 1.5–2 µm below the collarette, integrated, terminal, extending percurrently with 1–10 proliferations, and sympodially with 1–4 lateral openings, cylindrical, pale brown to subhyaline, with persistent remnants of the collarettes; collarettes 3.5–5 µm wide, 1.5–2.5 µm deep, funnel-shaped, dark brown becoming hyaline upon aging. Conidia 11.5–17.5(–18) × 2–3 µm (mean ± SD = 14.9 ± 1.1 × 2.5 ± 0.4 µm), falcate, setulae 4.5–7 µm long, accumulate in slimy whitish fascicles. Teleomorph. Unknown.
Specimens examined: CZECH REPUBLIC, Central Bohemian region, Úvaly, Škvorecká obora-Králicˇina Nature park, on decaying acorn of Quercus sp., 21 April 2018, M. Réblová M.R. 3944 (PRA-20991, culture CBS 146619); Ibid., M.R. 3946 (PRA-20992, culture CBS 146620); Ibid., M.R. 3948 (PRA-20993, culture CBS 146621); Ibid., South Bohemian region, Novohradské hory Mts., Horní Stropnice, Bedrˇichovský potok Natural Monument, on decaying acorn of Quercus sp., 5 October 2018, M. Réblová M.R. 3983 (PRA-20990). FRANCE, Ariège, Pyrenées Mts., Rimont, La Maille brook, alt. 550 m, on decaying acorn of Quercus sp., 28 May 2018 (incubated for 2 weeks in a damp chamber), J. Fournier M.R. 3951 (PRA-20994, culture CBS 145099); Ibid., M.R. 3952 (PRA-20995, culture CBS 145100). ITALY, on branch of Quercus suber, F. Marras (culture CBS 115959). JAPAN, Chiba Prefecture, Kiyozumi, on dead leaves of Lithocarpus edulis, Aug. 1954, K. Tubaki (authentic strain of Menispora minuta MUCL 9903 = MUCL 15759 = CBS 280.59). THE NETHERLANDS, Gelderland province, Rheden, National Park Veluwezoom, on decaying acorn of Quercus sp., 6 September 1969, V. Holubová-Jechová (CBS 966.69); Ibid., Utrecht province, Utrecht, Utrecht Science park near Westerdijk Fungal Biodiversity Institute, on a fallen leaf of Quercus sp., August 2018, M. Hernández-Restrepo M.H.R. 18063 (culture CBS 147518). USA, Louisiana, New Orleans, Audobon Park, on leaf litter of Quercus virginiana, 19 April 1974, R. Bandoni & K.A. Pirozyn- ski 6120 B/K.A.S. 7210 (culture DAOM 148141). USA, Texas, Houston, E. & A. Taylor Park, on a dead leaf of Quercus virginiana, date unknown, G. Bills (culture TTI-0830). USA, West Virginia, Fayette Co., Fayette Station, hardwood leaf litter, date unknown, collector unknown (culture ATCC 20960 = MF5247 = Mycosearch 1673).
Habitat and geographical distribution: Codinaeella minuta is a saprobe on fallen leaves and woody fruits of Fagus sylvatica, Lithocarpus edulis, Quercus petraea, Q. robur, Q. virginiana, Quercus sp., and other unknown hosts. It occasionally occurs on bark and wood of Q. suber. It is known from Asia, Europe, and North America: the Czech Republic, France, Japan, Italy, The Netherlands, Slovak Republic and USA ([26,174], this study).
According to GlobalFungi, the identical sequences were found in 20 samples of soil (80%), litter (15%) and root (5%) from forest (95%) or grassland (5%) habitats in Europe (60% of all samples, Denmark, France, Hungary, Poland, Sweden), Asia (15%, Iran), and in western part of North America (25%, Florida, Michigan). The samples originate from eight studies and the locations have temperate or humid continental climate (MAT avg. 13 ◦C, MAP avg. 864 mm). Codinaeella minuta is identical with Ca. filamentosa in ITS1 barcode. Thus, only ITS2 region was used to study C. minuta biogeography.
Notes: This species was described as Menispora minuta from dead leaves of Lithocarpus edulis, an evergreen tree native to Japan [174]. Although Tubaki [174] characterized this species in culture only, we update its description based on observations from nature based on the newly acquired material. Among members of Codinaeella, Ca. minuta is well distinguished by the production of deep orange pigment on nutrient media, especially on MLA and OA (Figure 28, Supplementary Files: Figures S3 and S4). Codinaeella minuta is similar to Ca. lutea, but it differs in shorter setae, slightly wider conidia and colony characteristics in vitro. For a detailed comparison, see notes to Ca. lutea.

Figure 27. Codinaeella minuta. (A–D,I–M) Conidiophores (E,N) conidia (F–H) colonies. Images: (A–E) from nature (F–H) on MLA after 4 weeks (I–N) on a stem of U. dioica on CMA after 4–8 week (A–E) from CBS 145099 (F) from CBS 146619 (G,H,L,M) from DAOM 148141 (I–K,N) from CBS 146619. Scale bars: (A–E,J–N) 10 µm; (F–H) 1 cm; (I) 20 µm.
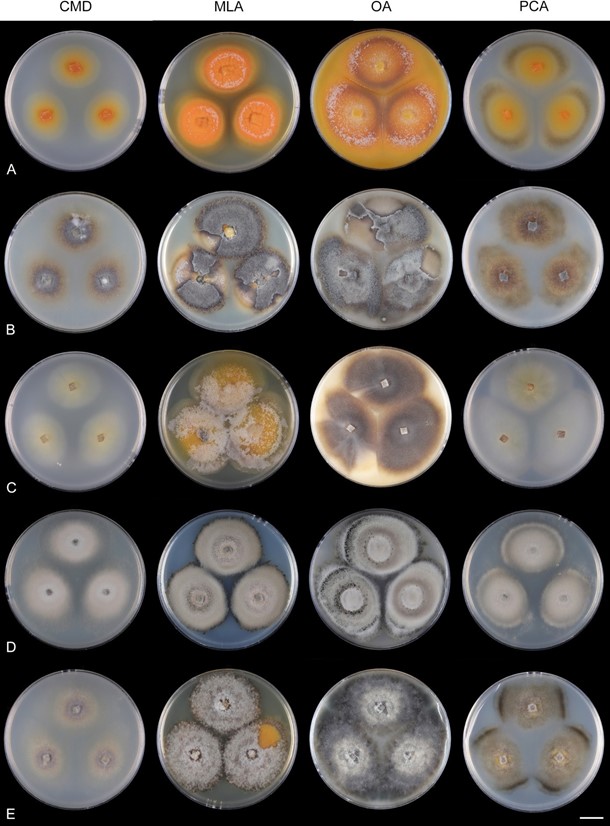
Figure 28. Colony morphology of Codinaeella minuta after 4 weeks. (A) CBS 966.69 (B) CBS 146621 (C) CBS 115959
(D) DAOM 148141 (E) ATCC 20960. Scale bar: (A–E) 1 cm.
