Babosia variospora D.G. Knapp, Zagyva, Trappe & Kovács
MycoBank nuumber: MB 834979; Index Fungorum number: IF 834979; Facesoffungi number: FoF14394;
Typification: HUNGARY. KISKUNSÁG: Semiarid sandy open grassland near Fülöpháza, 46°52′N, 19°25′ E, 23 Nov 2017, I. Zagyva, P. Vági, D.G. Knapp KIPD_02 (ZI1368) (holotype BP111127). GenBank: ITS = MN857190; 28S = MN857210; tef1 = MN882328; rpb1 = MN886304.
Etymology: variospora (Latin), in reference to the variability of spore ornamentation.
Diagnosis: Characterized by the dark sporogenous zone and dark brown spore color at maturity, the clear peridermal layer at maturity, and the variability of the ornamentation of the ascospores. These features distinguish the species from others known in the clade formed by Hydnobolites, Stouffera, and Temperantia in the Pezizaceae.
Description: Ascomata gasteroid, hypogeous, as fresh up to 25 mm broad, globose or subglobose (FIG. 1C, D). Peridium up to 1.5 mm thick, light gray to cream. Gleba solid, fleshy, dark brown in youth and also at maturity, with pockets of fertile tissue separated by sterile tramal veins partly empty to filled by opposing hymenia (FIG. 1C, D). Taste and odor not recorded. Ascopores in water pigmented, globose to subglobose, spinulate with spines of varying lengths or reticulate, 13.5–23 µm with ornamentation; rare spikes 2–4(–6) µm long, dense spines 1–2 µm long; wall of the reticulum 1–1.5(–2.5) µm thick (FIG. 4). Asci disintegrating with age, randomly arranged in fertile pockets in gleba of the ascomata, hyaline, slightly ellipsoid or saccate, 10–14 × 16–20 μm, nonamyloid, thin-walled, mostly with (4–)6 spores. Paraphyses lacking. Peridium plectenchymatic with a compact, pigmented, thinner (45–60 µm) outer layer and a hyaline thickened (250–300 µm) inner layer with some scattered cells inflated up to 30–45 μm wide (FIG. 4F).
Ecology and distribution: Sandy grasslands of the Great Hungarian Plain, in open mixed woody steppe, in certain locations dominated by Festuca and Stipa as dominant grasses, ascomata near nonectomycorrhizal herbaceous plant species such as Artemisia campestris, Corispermum nitidum, or Polygonum arenarium. Jun to Nov.
Additional material examined: HUNGARY. KISKUNSÁG: Semiarid sandy open grassland near Fülöpháza, 46°52′N, 19°25′E, 23 Nov 2017, I. Zagyva, P. Vági, D.G. Knapp KIPD_01; ibid., KIPD_03; semiarid sandy open grassland near Szabadszállás, 19 Oct 2017, I. Zagyva ZI_K (ZI1347); ibid., ZI_N; semiarid sandy open grassland near Tatárszentgyörgy, 7 Nov 2017, ZI_171107_1 (ZI1353); ibid., ZI_171107_1 (ZI1354); semiarid sandy open grassland near Csévharaszt, 1 Nov 2016, L. Zagyva, I. Zagyva G19 (ZI1248); 19 Jun 2018, ZI_180619_1; ibid., ZI_180619_2.
Notes: Ascomata of Babosia variospora can be found deeper than 5–10 cm below the soil surface and as deep as 40–50 cm. None of the ectomycorrhizal plant species of the habitat (Fumana procumbens, Populus alba, Salix rosmarinifolia) were found near the fungus. The ITS sequence of the fungus B. variospora was found in our database of the ITS-based metabarcode study of the soil fungal community of the grassland at Fülöpháza (data not shown; Vajna, Knapp, Kovács results). The fungus was not found/identified during a comprehensive ectomycorrhizal root tip– based study of the ECM fungi of F. procumbens, P. alba, and S. rosmarinifolia and the invasive Pinus nigra of the grassland near Fülöpháza (data not shown; see details on sampling in Seress et al. 2016).

Figure 1. Sampling site and ascomata of the truffles described in the paper. A. The main collection site, a sandy grassland near Fülöpháza. B. Ascomata of Stouffera gilkeyae in sandy soil. C. Ascomata of Babosia variospora (KIPD_01). D. Ascomata of B. variospora (KIPD_02). E. Ascomata of S. gilkeyae (KIPD_05). F. Damaged part of the ascomata of the holotype KIPD_07 showing gray discoloration. Bars: C–F = 1 cm.
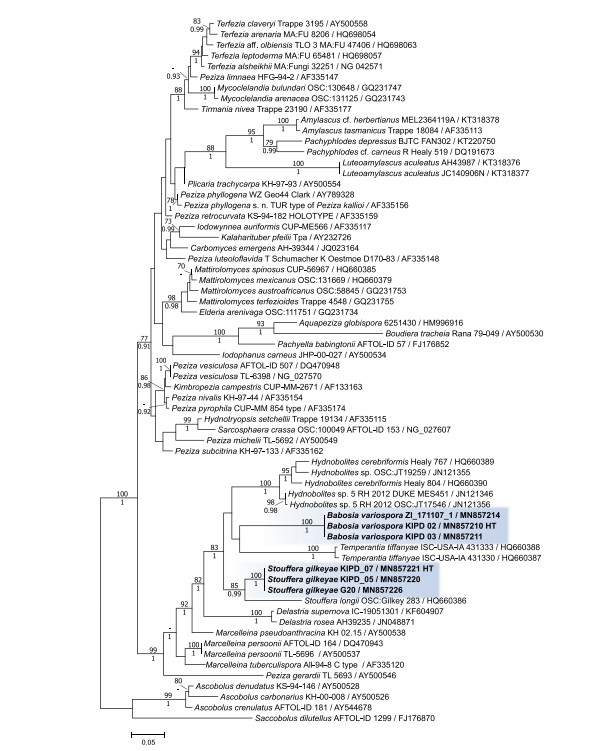
Figure 2. ML phylogenetic tree of 28S sequences of the two novel species and representative taxa of Pezizaceae. Bayesian posterior probabilities ≥0.90 are shown below branches; ML bootstrap support values ≥70% are shown above branches. Names of tips are followed by strain numbers and GenBank accession numbers separated by slashes. Sequences derived from holotype materials of the fungi introduced here are indicated as HT. The scale bar indicates 0.5 expected changes per site per branch.
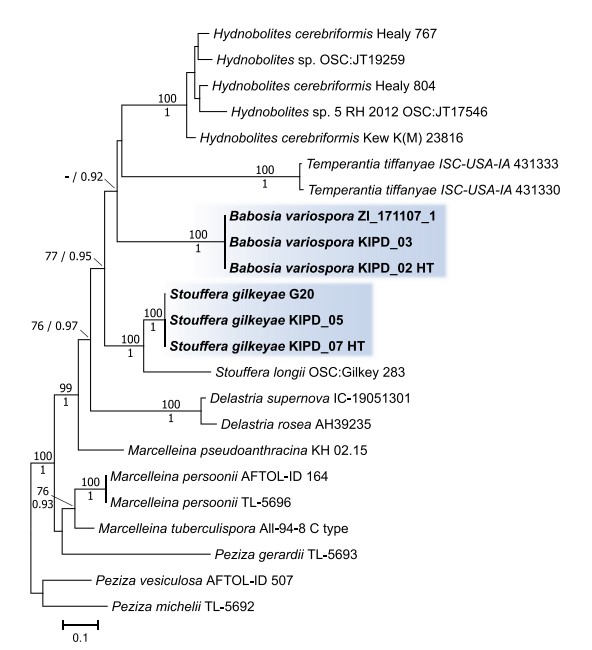
Figure 3. ML phylogenetic tree of combined ITS, 28S, and tef1 sequences and coded indel regions of the ITS and 28S region of the two novel genera and related taxa representing the clade of Pezizaceae where the new taxa grouped into. Bayesian posterior probabilities ≥0.90 are shown below branches or after slashes; ML bootstrap support values ≥70% are shown above branches or before slashes. Sequences derived from holotype materials of the fungi introduced here are indicated as HT. The scale bar indicates expected changes per site per branch.
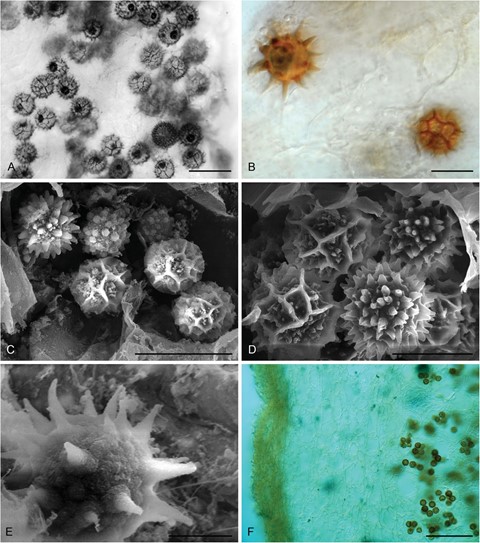
Figure 4. Micromorphology of Babosia variospora (holotype). A–B. Variously ornamented ascospores by light microscope. C–D. Scanning electron micrographs of diverse spores in asci of B. variospora. E. Spiny ascospore in the gleba. F. Peridium structure of B. variospora. Bars: A, C = 20 μm; B, D = 10 μm; E = 5 μm; F = 100 μm.
