Rhagadodidymellopsis endocarpi Fdez.-Brime, Gaya, Llimona & Nav.-Ros., sp. nov. (Figs 1–3)
MycoBank number: MB 835455; Index Fungorum number: IF 835455; Facesoffungi number: FoF;
Type: Spain, Catalonia, Girona, el Port de la Selva, punta de S’Arenella, 42°21′N, 3°10′E, open siliceous soil crusts mixed with isolated schist rocks, 5 m, on Endocarpon pusillum, 4 June 2011, S. Fernández-Brime 1155 & X. Llimona (BCN-Lich. 18949 – holotype).
Description and diagnosis. A lichenicolous fungus not producing any apparent damage to the host. Vegetative hyphae not distinct. Ascomata stromatic, perithecioid, pseudothecia-type, black, superficial, with an irregular and markedly coarse surface, growing on the surface of the host squamules; young stromatic 150–300 µm in diam., ± globose, unilocular, scattered on the host thallus, older stromatic ascomata 400–800 µm in diam., irregularly flattened, grouped (up to 10 observed). Cells of the basal part of the stroma forming a continuum with the cells of the host cortex, from which they can only be distinguished by the colour of the cell walls, dark brown in the stroma and hyaline in the cortex; cells of the wall irregularly rounded to elongate when tangentially sectioned, mostly (5–)7–11(–16) × (3.5–)4–8 µm, wall pigments impregnating the cell wall, not forming granules, turning black with K. Ascomal wall pseudoparenchymatous, of textura angularis, blackish brown in both the upper and lower parts; wall in small unilocular ascomata 35–80 μm thick in section, multi-layered, uniform in colour throughout their thickness, with the cell lumina of the external layers somewhat larger than those of more internal layers. Hymenial gel I–, K/I–. Interascal filaments abundant, branched and anastomosing, 1.5–2 µm wide. Ostiolar filaments visible near the ostiolar channel, 8–13 × 1–1.5 µm. Asci (4–)8-spored, (50–)60–75 × 13–16 µm (n = 12), with the ascus wall thickened near the apex, with a distinct ocular chamber, clavate, stipitate, with ascospores distichously arranged; endoascus I–; dehiscence fissitunicate. Asco- spores hyaline, narrowly obovate to elongated ellipsoid, with a smooth surface and a conspicuous gelatinous sheath (up to 1.5 µm thick); only in some mature ascospores can finely granulose ornamentation be observed on the sur- face, 1-septate, markedly constricted at the septum, lower cell narrower than upper cell and attenuated, with one or two oil droplets per ascospore cell, (13–)15.5–17.1– 19(–20.5) × (5.5–)6–7.0–8(–8.5) µm [length/width ratio: (2.0–)2.1–2.5–2.7(–3.4)] (n = 69). Conidiomata not seen.
Etymology. The ‘endocarpi’ refers to its host, the lichen genus Endocarpon.
Ecology and distribution. Up to date, only four col- lections from three localities of Rhagadodidymellopsis endocarpi are known. The type material was collected in north-eastern Catalonia (Spain) from several specimens of E. pusillum growing on siliceous clay soil in sun-exposed areas. Unlike other species living in the same locality, such as Epiphloea terrena and Gyalideopsis athalloides, Endocarpon pusillum is not ephemeral and does not become inconspicuous during dry periods. The other two samples were collected in Canberra (Australia), and also grow on typical E. pusillum.
Notes. In the genera Didymellopsis and Zwackhiomyces, the ascomata are usually produced solitarily, and the ascomatal wall thickness is uniform or slightly thicker towards the ostiole. Rhagadodidymellopsis endocarpi, in contrast, has the ascomata mostly grouped in stromata, and the ascomatal wall varies in thickness around the grouped pseudothecia, resulting in a markedly rugose excipular surface.
Didymellopsis perigena also grows on Verrucariaceae species with a squamulose thallus. This species differs from R. endocarpi by having solitary, non-stro- matic perithecioid ascomata (150–240 µm in diam.; fide Grube & Hafellner 1990), an ascomal wall of rather constant thickness, 30–65 µm wide, longer ascospores, 18–25 × 6–8.5 µm, with an average length/width value of ~3, and by growing mostly on the squamule margins of Placidium squamulosum (Grube & Hafellner 1990; Khodosovtsev & Klymenko 2015; see Table 1). Grube & Hafellner (1990) mentioned that D. perigena could grow not only on Placidium but also on Endocarpon, based on a record of ‘Didymella’ perigena from Nice (France) cited in Vouaux (1913). In Vouaux’s study, the presence of flattened ascomata and ascospores of 15–21 × 6–8 µm are mentioned. These features are fairly similar to those observed in R. endocarpi. However, it is also stated that the ascomata grow in the margin of the lichen squamules, which corresponds to the typical growth of D.perigena on the host thallus. Unfortunately, we were not able to locate Vouaux’s specimen and therefore cannot determine whether the ‘Didymella’ perigena specimen described by Vouaux (1913) belongs to R. endocarpi or to D. perigena. More recently, Yazıcı & Etayo (2015) reported D. perigena in Turkey, growing on Endocarpon cf. pusil- lum. We revised this Turkish specimen and it matches well the description of D. perigena. There is a further citation of D. perigena from Cabo Verde (van den Boom 2012), potentially growing on E. pusillum, but as the author did not include morphological data regarding the lichenicolous fungus we cannot determine whether it corresponds to the newly described R. endocarpi or to D. perigena.
An additional ecological observation is that the irregularities on the surface of the stromata of Rhagadodidymel- lopsis endocarpi are always colonized by cyanobacteria, while no cyanobacteria are observed on lichen squamules devoid of the lichenicolous fungus. Based on this observation, we speculate that R. endocarpi might have a habit similar to the one mentioned by Grube & Hafellner (1990) in D. perigena: these authors hypothesized that as D. perigena was the only species from the genus not growing on cyanolichens, it could establish symbiosis with cyanobacteria accumulated at the base of the ascomata and using the lichen only as a mere substrate. If this was the case, the same relationship could occur in R. endocarpi, which would exploit the cyanobacteria accumulated in the irregularities of the stromata.
Additional specimens examined. AUSTRALIA. Australian Capital Terr.: Cotter Reserve, ~20 km W of Canberra, ~500 m, 10 August 1988, H. Mayrhofer 8945 & H. Streimann (GZU, Inv. Nr. 12-PO); Latham, 12 km NW of Capital Hill, Canberra, flat grassy verge beside the road, on bare semi-shaded ground, 35º13’S, 149º02’E, ~560 m, 9 August 1993, H. Streimann 51974 (NY). SPAIN. Catalonia, Girona, El Port de la Selva, Punta de s’Arenella, open siliceous soil crusts with isolated schist rocks, 42°21’N, 3°10’E, 5 m, 4 June 2011, S. Fernández-Brime 1156 & X. Llimona (BCN-Lich. 18950).
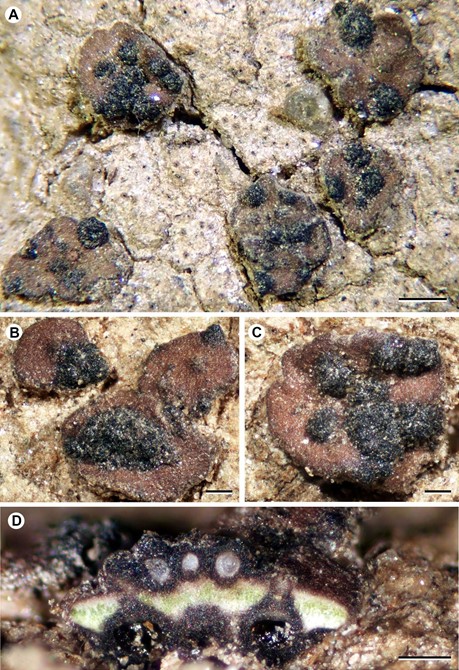
Figure 1. Rhagadodidymellopsis endocarpi (BCN-Lich. 18949, holotype). A–C – host thallus with R. endocarpi superficial ascomata; D – cross section of stroma-grouped ascomata. Scales: A = 500 μm; B–D = 200 μm.
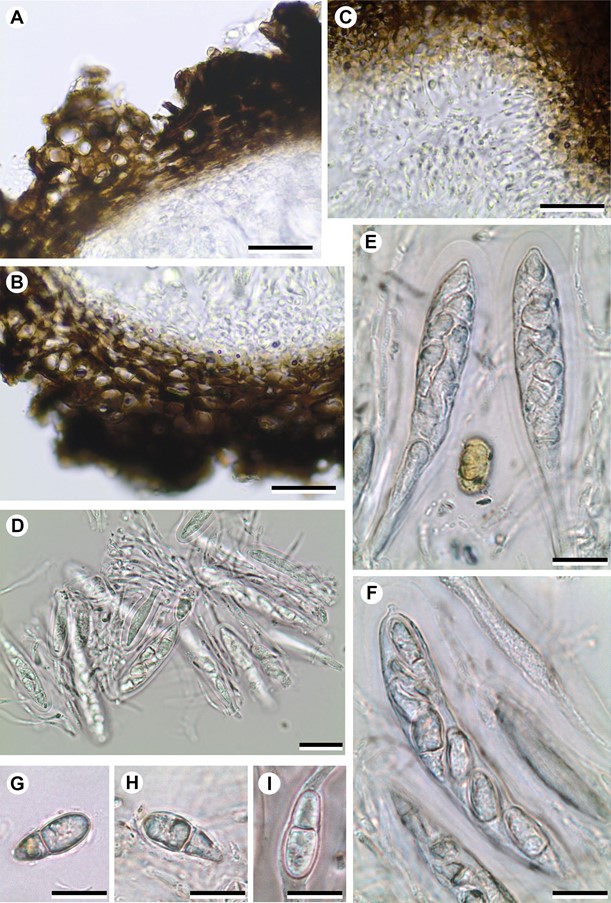
Figure 2. Rhagadodidymellopsis endocarpi (BCN-Lich. 18949, holotype) A–B – ascomal wall in cross section; C – ostiolar filaments close to ostiolar canal; D – asci and interascal filaments; E–F – asci with ascospores; G–I – ascospores. All microscopy sections mounted in water. Scales: A–D = 20 μm; E–I = 10 μm.
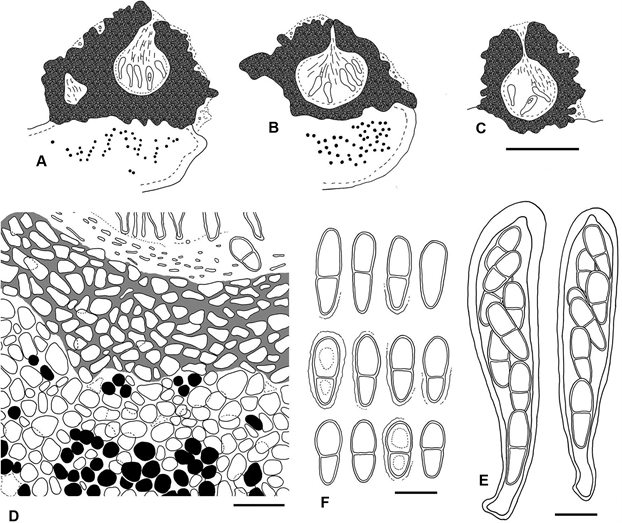
Figure 3. Rhagadodidymellopsis endocarpi (BCN-Lich. 18949, holotype) A–C – schematic sections of ascomata; D – basal part of ascomal wall (pigmented) forming a continuum with upper cortical layer of host (non-pigmented); E – asci with ascospores; F – ascospores. Scales: A–C = 200 μm; D = 20 μm; E–F = 10 μm.
