Lichenoconium hawksworthii Flakus, Etayo, Kukwa & Rodr. Flakus, sp. nov. (Fig. 12)
MycoBank number: MB 838684; Index Fungorum number: IF 838684; Facesoffungi number: FoF 10424;
Holotype: A. Flakus 27364 (KRAM).
Etymology. The new species is named in honour of the Professor David L. Hawksworth, eminent British mycologist and lichenologist, for his great contribution to our knowledge of lichenicolous asexual taxa and also to the Lichenoconium genus.
Diagnosis: Differs from Lichenoconium aeruginosum by having larger conidiomata, 150–250 × 70–230 μm, producing purple pigment in apical and lateral part of exciple becoming strongly aeruginose in KOH, and smaller conidia, 3–4.5 × 2.5–4 μm.
Description: Mycelium indistinct, completely immersed in the host, composed of hyaline to pale brown hyphae, septate, uneven in thickness, about 2–3 µm thick. Sexual morph: Undetermined. Asexual morph: Conidiomata pycnidial, arising singly or in groups, at maturity the uppermost part evidently exposed, convex, black and matt, subglobose to obpyriform, (150–) 200–250 µm high, (70–)150–200(–230) µm wide, ostiole conspicuous, apical, covered by accumulating, dark-brown conidial mass. Conidiomatal wall pseudoparenchymatous composed of 3–6 layers of cells, 8–25 µm thick, with cells irregularly polyhedral to almost subglobose in shape, lumen 3–10 × 2–4 µm. Wall thickened in the upper part, pale to dark brown below and dark purple in the upper and lateral part, apical part usually incrusted by hyaline crystals, the purple pigment located in the exciple becoming strongly aeruginose in KOH. Conidiogenous cells forming a single layer lining the pycnidial cavity, subcylindrical, phialidic, with visibly narrower necks, hyaline, (6–)9–12 × (2–)2.5–3.5 µm. Conidia arising from the apices of the conidiogenous cells, abundant, accumulating in a dry mass in the conidiomatal cavity, globose to subglobose or rarely slightly obovoid, not attenuated, apically rounded, basally sometimes narrower, the base usually truncated, yellow-brown to dark-brown, almost black in mass, delicately warted, aseptate, (3–)3.5–4(–4.5) × (2.5)3–3.5(–4) µm.
Material examined: Bolivia, Dept. Tarija, Prov. Burnet O’Connor, close to Entre Ríos, new road between Tarija and Entre Ríos, 21º30’47”S, 64º11’49”W, 1338 m, disturbed Tucumano-Boliviano forest with shrubs and Tillandsia, on thallus of Heterodermia comosa, 28 July 2015, A. Flakus 27364 (KRAM27364, holotype, LPB – isotype). GenBank No.: MW315198
Additional specimens examined: Lichenoconium cf. cargillanum: Bolivia, Dept. Tarija, 22º02’38”S, 64º35’47”W, on apothecial disc of Punctelia sp., 2015, A. Flakus 27023 (KRAM, LPB); GenBank No.: MW315196; Lichenoconium erodens: Bolivia, Dept. Chuquisaca, 18º45’53”S, 64º49’57”W, on thallus of Parmotrema retuculatum, 2015, A. Flakus 26452 (KRAM, LPB); GenBank No.: MW315197.
Ecology and distribution: Lichenoconium hawksworthii is known from the type locality in Tucumano-Boliviano forests in Bolivian Andes and grows on epiphytic Heterodermia comosa.
Notes: The phylogenetic placement of Lichenoconium in Dothideomycetes was shown by Lawrey et al. (2011). Ertz and Diederich (2015) recently found that Lichenoconium is closely related to Abrothallus, however, to fully understand the phylogenetic relationship between those two genera additional analyses with larger datasets are necessary.
The new species occurs on discolored or brownish infected parts of the host thalli, and is characterized by only partly immersed, large conidiomata, large conidiogenous cells, small conidia and the presence of purple pigment in apical and lateral part of exciple becoming strongly aeruginose in KOH. The two most morphologically similar species are Lichenoconium aeruginosum and L. pyxidatae growing mainly on Cladonia. Lichenoconium aeruginosum differs in smaller conidiomata, 80–100(–140) μm diam., with bluish pigment (turning aeruginose in KOH), and larger conidia, (3.4–)3.8–4.6(–5.4) × (3.0–)3.4–3.8(–4.3) μm. Lichenoconium pyxidatae also differs in smaller conidiomata, (60–)120(–150) μm diam., with brown pigment in exciple (becoming darker and olivaceous in KOH), and slightly smaller conidia, (2–)2.5–3.5(–4) × 2–3 μm (Hawksworth 1977, Cole & Hawksworth 2004, Lawrey et al. 2011).
Our phylogenetic analyses based on nuLSU sequences (Fig. 13) show that L. hawksworthii is placed in a highly supported clade that includes six species of Lichenoconium. Although we included in our analyses all Lichenoconium sequences available in GenBank, the relationship of our new species to other species within the genus remains uncertain due the lack of statistical support. However, it seems to place together with the Bolivian sample of L. cf. cargillanum (growing on apothecial discs of Punctelia). The significant phylogenetic distance between L. hawksworthii and L. aeruginosum together with morphological discrepancies and different host preferences seems to justify the description of the new species.
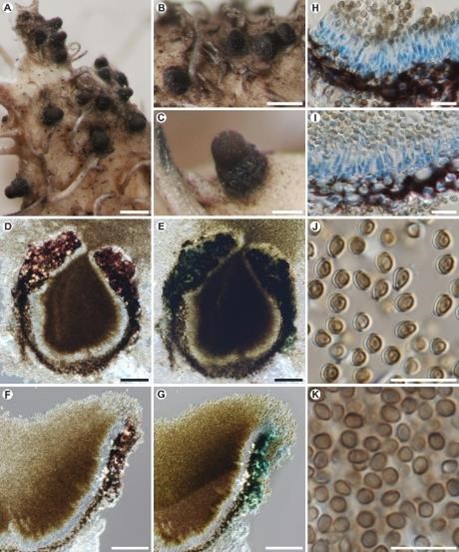
Figure 12 – Lichenoconium hawksworthii (holotype). A–C, Habit of conidiomata growing in thallus of Heterodermia comosa. D–E, Transversal section of conidiomata showing excipular pigmentation and conidial mass filling the conidiomatal cavity (D in water, E in KOH), F–G, Transversal section of exciple (F in water, G in KOH), H–I, Conidiogenous cells (in LPCB), J–K, Conidia (J in water, K in LPCB). Scales: A–B = 250 µm, C = 100 µm, D–E = 50 µm, F–K = 10 µm.
