Ericiomyces syringoforeus sp. nov. Karpov et Reñé
MycoBank number: MB 836467; Index Fungorum number: IF 836467; Facesoffungi number: FoF;
GenBank numbers: MT998435 (18S rDNA), MT998437 (ITS), MT998436 (28S rDNA). Figures 2, 3, 4, 5, 6, 7.
Parasitoid of dinophytes, with some preference for Kryptoperidinium species. Mature epibiotic spherical spiny sporangium of 20–25 μm in diameter with smooth lateral papilla. Spines are up to 1.5 μm in length. Spherical zoospores of 3.9–4.8 μm in diameter with a posterior lipid globule, flagellum is 26–27 μm. Zoospores are released through a dis- charge pore appr. 10 μm in diameter. Syringe-like structure is 1.2 μm long.
Etymology. From the Greek σύριγγα syringa – syringe, and φορέας foreus – carrier, the unique organelle for infection.
Type strain: E4, isolated on the host Kryptoperidinium foliaceum from samples collected in Kökar, located in Åland Archipelago (SW coast of Finland) in the northern Baltic Sea in June 2016.
Holotype: a fixed specimen derived from the strain E4 embedded in a resin block for electron microscopy deposited in the CCPP ZIN RAS under the No X-135.
Ecology: Ericiomyces syringoforeus appeared in samples obtained during a bloom of the dinoflagellate Kryptoperidinium foliaceum. Concurrently, other parasites were present in those samples infecting the same host, e.g., Parvilucifera catillosa, an endoparasitoid belonging to the Perkinsozoa (Alveolata) (Alacid et al. in press). Thus, fungal parasitism is not the only factor involved in the changes of population dynamics of the dinoflagellate, which can be attacked and affected by different co-occurring parasites. In both cases, infections were observed after the incubation of the natural samples in the laboratory and to the best of our knowledge, it represents the first record of co-occurring chytrid-perkinsid species simultaneously infecting the same host. Unfortunately, the prevalence of each species in the natural population could not be determined but cross-infection experiments showed the chytrid was able to infect efficiently K. foliaceum, as well as H. triquetra, while infections were not observed on other phytoplankton species tested. Those same host species were tested for infections of P. catillosa (Alacid et al. in press), showing exactly the same results on susceptibility. Thus, it confirms that both parasites are competing for the same resources. Cross-infection experiments were also performed for D. arenysensis (Lepelletier et al. 2014b). Only positive results were obtained for dinoflagellate representatives, being able to infect 31 different strains out of 48 tested, belonging to 13 different species. However, K. foliaceum was not infected by D. arenysensis. Even though there is a bias in the number of species tested in the cross- infection experiment, both species showed remarkable differences regarding their host preferences. Further studies should focus in the role of parasitism in dinoflagellate population dynamics and understanding those fluxes would help to elucidate the mechanisms of blooms development.
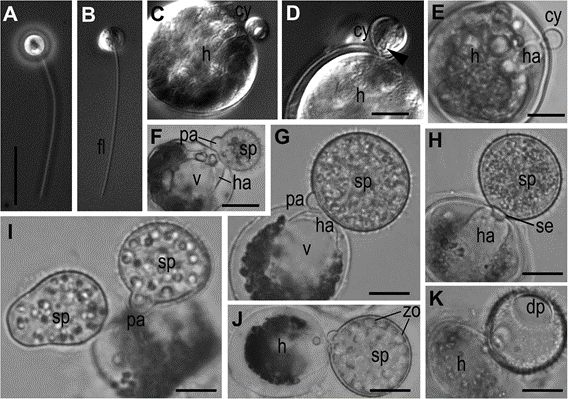
Fig. 2 Light microscopic images of the life-cycle stages of Ericiomyces syringoforeus. A, B—zoospores at Ph (A) and DIC (B); C—zoospore with retracted flagellum recently attached to the host (DIC); D—encysted zoospore with syringe (arrowhead) (DIC); E—encysted and germinated cell with haustorium (BF); F—young spiny sporangium with papilla; G, H—multinucleate immature sporangium with developed haustorium and septa (H); I, J—mature sporangia with formed zoospores (J); K—empty sporangium with a discharge pore after zoospore release. E–K—BF im- ages. Scales: A–C, E, G–K—10 μm, D—5 μm, F—15 μm
Abbreviations: am-amoeba, br-bridge, c-centriole, cy-cyst, dp-discharge pore, dv-dense vesicles, er-endoplasmic reticulum, fc-food cap, fl-flagellum, fu-funnel, h-host, ha-haustorium, k-kinetosome, l-lipid globule, m- mitochondrion, ma-microtubules of the axoneme, mi-microbody, mr- microtubular root, n-nucleus, ne-needle of the syringe, pa-papilla, pl- plate at the base of microtubular root, pr-props, rc-ribosomal core, s- spines, se-septa, sa-saddle, sp-sporangium, sr-subunits of ribosomes, sw-sporangial wall, sy-syringe, v-vacuole, zo-zoospore
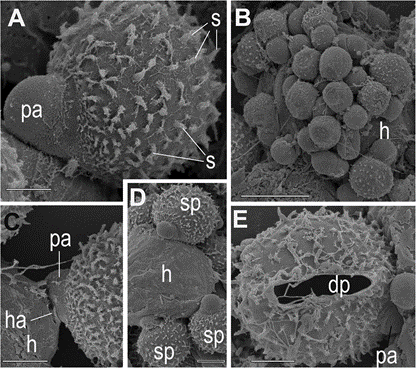
Fig. 3 Scanning electron microscopical images of sporangia and cysts of Ericiomyces syringoforeus. A— typical shape of spiny sporangium with papilla on the host surface; B—multiple infection of the Kryptoperidinium foliaceum cell (h), covered by cysts and early sporangia; C—haustorium penetration into the host; D—several growing spiny sporangia on host surface; E—empty sporangium with a discharge pore after zoo- spore releasing and papilla. Scales: A, C–E—5 μm, B— 10 μm
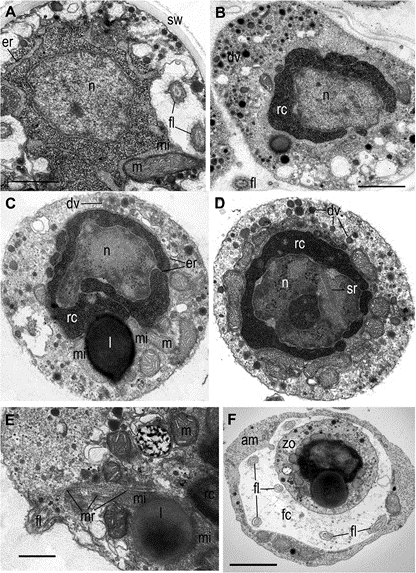
Fig. 4 General zoospore structure of Ericiomyces syringoforeus at ultrathin sections. A— intrasporangial nearly mature zoospore but still with dispersed ribosomes; B—intrasporangial mature zoospore with aggregated ribosomes; C, D—released zoo- spore structure; E—posterior end of released zoospore with flagellum and anterior microtubular root; F—zoospore in the food cup of heterolobosean amoeba. Scales: A—1 μm, B–D—1 μm, E—500 nm, F—2 μm
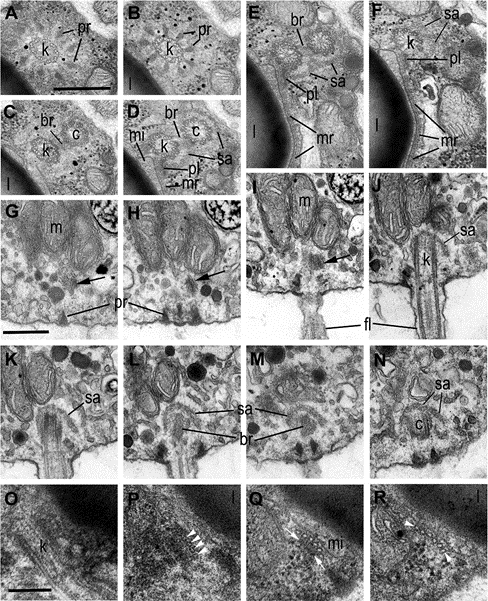
Fig. 5 Flagellar apparatus structure in zoospore of Ericiomyces syringoforeus. A– F—consecutive transversal sections of kinetosome and centriole in direction from distal to proximal end; G–N— consecutive longitudinal sections of kinetosome and centriole, arrows show oblique section of microtubular root with plate; O– R—consecutive transversal sections of microtubular root (bars) and its basal plate (arrowheads) in direction from its origin at kinetosome to distal end. Scales: A–F—400 nm, G–N— 400 nm, O–R—150 nm
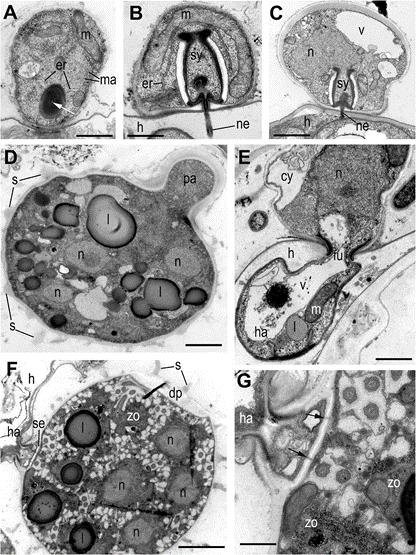
Fig. 6 Cyst and sporangia structure of Ericiomyces syringoforeus at ultrathin sections. A—recently encysted zoospore with lipid globule containing a tube in the center (white arrowhead); B—cyst with mature syringe penetrating the host wall. C—part of sporangium near the papilla with thickened wall and mature syringe penetrating the host wall; D—immature multinucleate sporangium with papilla; E—sporangium with rhizoid fixed in the host cell wall by funnel structure; F—mature sporangium with zoospores septa, funnel and discharge pore; G—structure of septa at higher magnification. Arrows show the pores in septa. Scales: A—800 nm, B—500 nm, C—1 μm, D—2.5 μm, E—800 μm, F—2 μm, G—500 nm
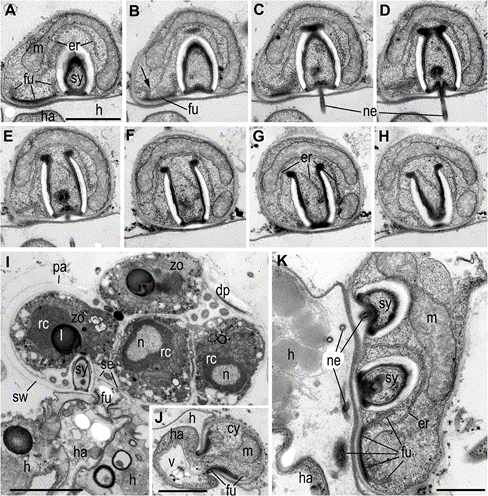
Fig. 7 Structure of syringe of Ericiomyces syringoforeus at the ultrathin sections. A–H—serial longitudinal sections of syringe in the cyst. Arrow on B shows ER connection with funnel. I— sporangium with mature zoo- spores contains an old syringe and funnel with haustorium. J—funnel-shaped structure in the cyst. K—part of young sporangium with two syringes and penetrative funnel. Scales: A–H—1 μm, I— 2 μm, J, K—400 nm
