Cryolevonia sChafbergensis SP. NOv. A. PONTES, J. RuEThI, B. fREy, A. AIRES, A. ThOMAS, D. OvERy, B. hAlTI, R. KERR & J. P. SAMPAIO
Cryolevonia schafbergensis (schaf.berg.en’sis. N.L. fem. adj. schafbergensis pertaining to Schafberg, the name of a moun- tain area located up to Pontresina in the upper Engadine valley in Switzerland, where the type strain was isolated).
MycoBank number: MB 831395; Index Fungorum number: IF 831395; Facesoffungi number: FoF;
The GenBank/EMBL/DDBJ accession numbers of the LSU and ITS sequences of Cryolevonia schafbergensis sp. nov. PYCC 8347T are MN058075 and MN058074, respectively.
After 2 weeks on YM agar at 8 °C cultures have a light pink colour and have a butyrous texture (Fig. S3). After 2 weeks of growth on YM agar at 8 °C, cells are elongated (6–14×2–3 µm) (Fig. 2) and proliferation is done by budding at the distal ends of the cell. On Dalmau plates after 4 weeks at 8 °C, rudimentary pseudohyphae are present but true hyphae are not formed. On potato dextrose agar incubated at 8 °C for 8 weeks inconspicuous stretches of narrow hyphae of approximately 1 µm in diameter and devoid of clamp connections are formed. Occasionally globose to sub-globose teliospore-like structures measuring 6–10 µm in diameter can be observed on the hyphae. The induction of germination of the teliospore-like structures was unsuccessful, even after a prolonged resting stage of 12 weeks in demineralized water kept at 4 °C.
Glucose is not fermented. Carbon compounds assimilated: d-glucose, sucrose, maltose, salicin, melezitose, d-glucitol (delayed and weak or negative), glycerol (delayed), d-mannitol, d-gluconate (delayed or negative), succinate (delayed) and ethanol (delayed). Carbon compounds not assimilated: d-galactose, l-sorbose, d-glucosamine, d-ribose, d-xylose, l-arabinose, d-arabinose, l-rhamnose, trehalose, methyl α-d-glucoside, cellobiose, melibiose, lactose, raffinose, inulin, soluble starch, erythritol, ribitol, xylitol, galactitol, myo-Inositol, d-glucono-δ-lactone, d-glucuronate, dl- lactate, citrate, methanol, l-malic acid, l-tartaric acid and protocatechuic acid. Nitrogen compounds assimilated: nitrate and nitrite. Nitrogen compounds not assimilated: ethylamine, l-lysine, cadaverine, creatine and creatinine. No growth in the presence of 0.01% cycloheximide. Growth in the presence of 16% NaCl. Growth in the absence of vitamins. Strong growth at 4 °C and 11 °C, weak growth at 17 °C, no growth at 18 °C. Hydrolysis of urea and DBB reaction are positive.
The holotype (PYCC 8347 h) is permanently maintained in a metabolically inactive state in the Portuguese Yeast Culture Collection, Caparica, Portugal, and the type strain was deposited in the same collection (PYCC 8347T), in the collection of the Westerdijk Fungal Biodiversity Institute (CBS 16055T), Utrecht, The Netherlands and in the Culture Collection of Switzerland (CCOS 1156T). The MycoBank accession number is MB831395. The strain D/1/Z/414 (PYCC 8347T) was isolated in September 2014 from a layer of permafrost soil located at 160 cm from the surface and collected in the mountain ridge ‘Muot da Barba Peider’, south-west of Piz Muragl and north-east of Pontresina in the upper Engadine valley (Eastern Swiss Alps), at an altitude of 2979 m (GPS: 46° 29.780′ N, 9° 55.887′ E).
The salient physiological characteristics of the new species are, firstly, its cryophile profile being unable to grow at 18 °C or higher temperatures, and also its ability to grow in the presence of 16% NaCl and with nitrate and nitrite as sole nitrogen sources. Of note is also the narrow range of utilization of carbon sources, a relatively uncommon feature for basidiomycetous yeasts.
From the phylogenetic perspective, the genus Cryolevonia is related to Camptobasidium and Glaciozyma. Whereas Camptobasidium includes a single species that is strictly filamentous, colonizes freshwater habitats and produces transversely septate basidia in the absence of teliospores [11], Glaciozyma includes currently four species that are unable to grow at temperatures higher than 20–21 °C and that produce teliospores that germinate with yeast cells or hyphae and therefore a sexual stage is unknown [12]. Since the ITS sequence of Camptobasidium hydrophilum is not available, only LSU D1/D2 sequences can be used to analyse the phylogenetic relationships of these three genera, that appear to form a clade that corresponds to the family Camptobasidiaceae (Fig. S1). Our phylogenetic analyses revealed also that several close relatives of Cryolevonia are known only from DNA sequences obtained in metagenomic studies and cultures have, until now, not been obtained (Figs 1 and S2). This might suggest that the currently employed techniques for cultivation of yeasts of the Camptobasidiaceae during environmental surveys might not be optimal and, although being present in the samples, several representatives of this group might escape cultivation. It is also relevant that similarly with Glaciozyma, Cryolevonia produces teliospores that are not amenable to germination by forming a basidium. This aspect, which is uncommon in teliospore-producing basidiomycetes, and the phylogenetic proximity of the two genera, should be addressed in further studies.
Here we investigated an ancient high alpine permafrost in the Swiss Alps, that has been permanently frozen for around 12 000 years according to 14C-radiocarbon measurements [10]. In a previous study we employed a culture-independent approach that revealed that yeasts and lichenized fungi were highly enriched in the permafrost layer as compared to the nearby non-permafrost soils [10]. Several genera of basidiomycetous yeasts were prominent, namely Rhodoto- rula, Naganishia, Mrakia and Leucosporidium, as well as the ascomycetous lichens Lecidea, Acarospora and Umbilicaria. These fungal groups were also found to be common in other studies of permafrost-like habitats [5, 13], including also other cold-adapted yeasts [14]. In this study we were able to recover viable yeast cells from the 12 000 year old high alpine permafrost mentioned above, which should be regarded as an uncommon event if the results from other studies, normally focused on prokaryotes, are taken into consideration. Firsty, the viability of microbial cells in permafrost soils is low. For example, only 26% of the cells from a Spitsbergen permafrost were viable [15]. Secondly, several studies suggest that only a small proportion of the microbial community of permafrost is cultivable [16–18]. In addition, the isolation of another strain of the novel taxon from melted sea ice, together with other basidiomycetous yeasts draws attention to this unexplored habitat. Although sea ice contains a complex microbial community primarily sustained by photosynthetic prokaryotes but that also contains heterotrophic bacteria, protists and meiofauna such as nematodes, copepods, rotifers and polychaetes [19], fungi have not been the target of comprehensive ecological surveys. The results presented here suggest that sea ice constitutes a relevant niche for cryophile and cryotolerant yeasts.
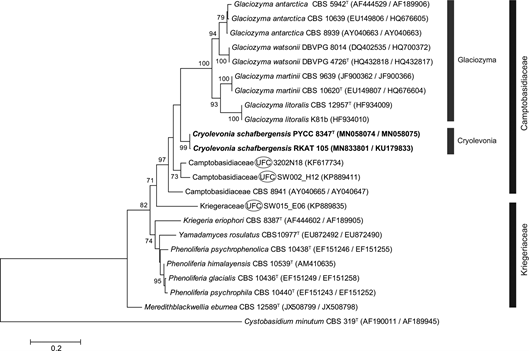
Fig. 1. Phylogenetic placement of Cryolevonia schafbergensis gen. nov and sp. nov. among the members of the families Camptobasidiaceae and Kriegeriaceae. The phylogenetic tree was based on a concatenated alignment of the D1/D2 domain of 26S rDNA and complete ITS region and used the maximum-likelihood method and the Tamura 92 model of sequence evolution. The tree was rooted with Cystobasidium minutum and environmental sequences for which cultures are not available are labelled as UFC (uncultured fungal clone). The numbers provided on branches are frequencies with which a given branch appeared in 1000 bootstrap replications (values below 70% not shown). The scale bar indicates the expected number of substitutions per site.
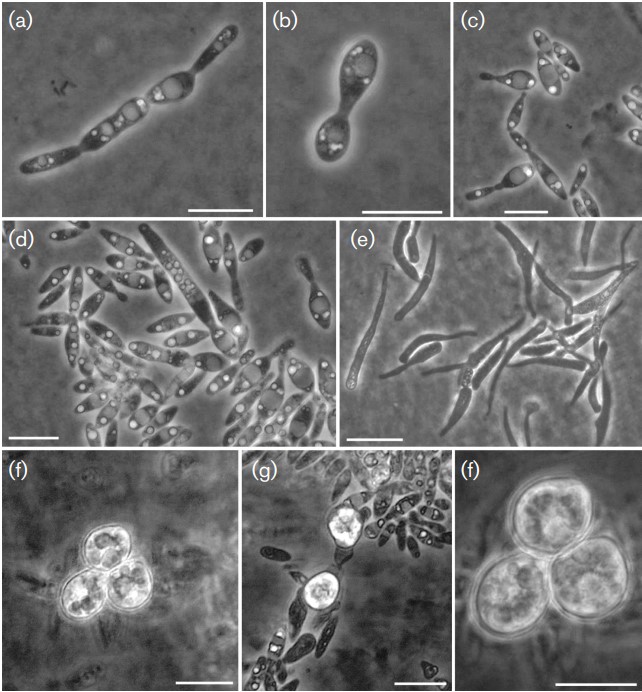
Fig. 2. Micrographs of Cryolevonia schafbergensis. (a–d) Yeast cells of PYCC 8347T on yeast–malt agar after 2 weeks at 8 °C and (e) after 10 months on corn meal agar incubated at 8 °C. (f–h) Teliospore-like structures formed on short hyphal stretches devoid of clamp connections on potato dextrose agar incubated at 8 °C for 2 months. Bars, 10 µm.
