Chaetosphaeria jonesii R.H. Perera, Maharachch., Camporesi & K.D. Hyde, in Perera, Maharachchikumbura, Bhat, Al-Sadi, Liu, Hyde & Liu, Mycosphere 7(9): 1311 (2016)
Index Fungorum number: IF 552574, Facesoffungi number: FOF 02659
Etymology – In honour of E.B.G. Jones for his work on tropical mycology.
Saprobic on wood. Sexual morph Ascomata 102–250 µm diameter, 142–263 µm high, superficial, arranged in clusters, subglobose to globose, dark brown, surface rough, ostiolate, surrounded by setae. Ostiole periphysate. Setae brown, multi-septate, sinuous, cylindrical, with a rounded apex, arising from the base of ascomata and abundant on the substrate. Peridium composed of dark brown cells of textura angularis in surface view, 11–16 µm thick in longitudinal section, 2-layered, inner layer 3–5 cells thick, composed of hyaline elongate cells of textura angularis, with outer layer 4–7 cells thick, composed of brown to dark brown, globose to polygonal cells. Paraphyses 3.9–4.6 µm wide, sparse, simple, septate. Asci 69–90×8.5–11 (x̅= 80×10 µm, n = 20) µm, 8-spored, unitunicate, cylindrical-clavate, short-pedicellate, with a thin, J-, apical ring. Ascospores 16.2–
17.7×2.8–3.6 (x̅= 19.5×3.6 µm, n = 20) µm, overlapping biseriate, hyaline to pale brown, fusiform, 3-septate, curved, smooth-walled. Asexual morph Undetermined.
Material examined – THAILAND, Chiang Mai, on decorticated wood, 5 August 2015, S. Boonmee, RHP 121 (MFLU 16-1020, holotype), ex-type living culture, MFLUCC 15-1015.
Notes – Maximum likelihood and Bayesian analyses of combined LSU, ITS and TUB sequence data shows Chaetosphaeria jonesii (MFLUCC 15-1015) clustered with the ex-type strain of C. tropicalis F.A. Fernández & Huhndorf (SMH 1267) which was earlier collected from Puerto Rico, on a wood fragment. We observed that the asci (69–90×8.5–11 vs. 100–138×10–12.5 µm; Fernández & Huhndorf 2005) and ascospores (16.2–17.7×2.8–3.6 vs. 19–26×3.2–6.3 µm; Fernández & Huhndorf 2005) of our strain are smaller than C. tropicalis. Chaetosphaeria jonesii is also phylogenetically and morphologically close to C. lateriphiala F.A. Fernández & Huhndorf and C. sylvatica F.A. Fernández & Huhndorf (Fig 1). However, the ascomata (102–250×142–263 µm), asci (69–90×8.5–11 µm) and ascospores (16.2–17.7×2.8–3.6 µm) of C. jonesii are smaller than those of C. lateriphiala (ascomata: 200–248×234–307 µm, asci: 95–113×10–12.5 µm, ascospores: 18–24×4.5–6 µm; Fernández & Huhndorf 2005). Chaetosphaeria jonesii also can be distinguished from C. sylvatica by its smaller ascomatal size (102–250×142–263 µm vs. 264–302×269–292 µm;
Fernández & Huhndorf 2005), smaller asci (69–90 µm vs. 95–115 µm; Fernández & Huhndorf 2005) and thinner ascospores (2.8–3.6 vs. 4–5.5; Fernández & Huhndorf 2005). By taking into account the morphological and molecular data, we introduce Chaetosphaeria jonesii as a new species.
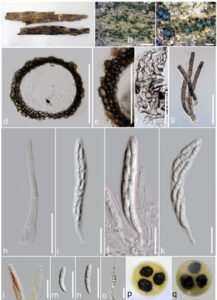
Figure 1 – Chaetosphaeria jonesii (MFLU 16-1020, holotype). a. Herbarium specimen. b, c. Appearance of ascomata on host substrate. d. Section of ascoma. e. Peridium. f. Peridium in surface view. g. Setae. h. Paraphyses. i, j. Immature and mature asci. k. Close-up of apical asci in Melzer’s reagent. l. Ascospores. m. Germinating ascospore. Bars – b, c = 2 mm, d = 100 µm, e = 50 µm, f–o = 20 µm.
