Pleurotheciales Réblová & Seifert, Persoonia 37: 63 (2015)
MycoBank number: MB 813228; Index Fungorum number: IF 813228; Facesoffungi number: FoF 06531;
Pleurotheciales was introduced based on a combined ITS, SSU, LSU, tub2, mcm7 and rpb2 dataset (Réblová et al. 2016c) and it presently accommodates Pleurotheciaceae which in turn comprises ten genera. Taxa belonging to Pleurotheciales cannot be successfully differentiated based on their overlapping sexual morphology. The type of conidiogenesis can, to a certain extent, delineate species into groups within the order, since conidial secession is rhexolytic or schizolytic with holoblastic, monoblastic or polyblastic (sympodially extending) conidiogenesis (Réblová et al. 2016c). Molecular data and/or cultures are essential to establish the placement of Pleurotheciales taxa (Réblová et al. 2016c). In this study, Pleurothecium obovoideum does not cluster with other Pleurothecium species, including the type species, P. recurvatum (Fig. 10). This result is in agreement with Réblová et al. (2012). The divergence time for Pleurotheciales is estimated as 105 MYA (Fig. 2), which is evidence of family status. The status as an order may need revision following further study. Currently there is one family and ten genera in this order (this paper).
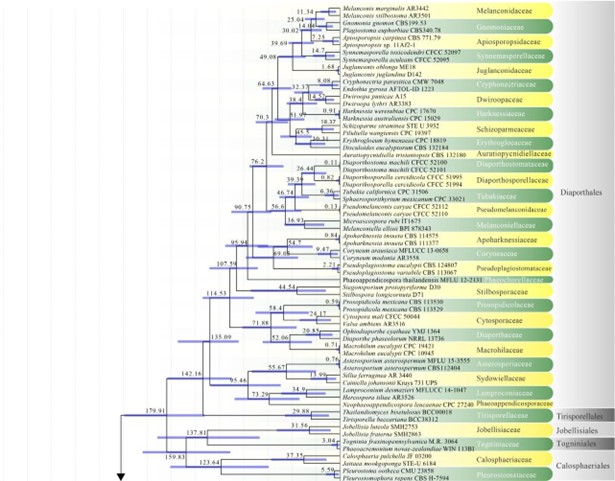
Figure 2 – The maximum clade credibility (MCC) tree, using the same dataset from Fig. 1. This analysis was performed in BEAST v1.10.2. The crown age of Sordariomycetes was set with Normal distribution, mean = 250, SD = 30, with 97.5% of CI = 308.8 MYA, and crown age of Dothideomycetes with Normal distribution mean = 360, SD = 20, with 97.5% of CI = 399 MYA. The substitution models were selected based on jModeltest2.1.1; GTR+I+G for LSU, rpb2 and SSU, and TrN+I+G for tef1 (the model TrN is not available in BEAUti 1.10.2, thus we used TN93). Lognormal distribution of rates was used during the analyses with uncorrelated relaxed clock model. The Yule process tree prior was used to model the speciation of nodes in the topology with a randomly generated starting tree. The analyses were performed for 100 million generations, with sampling parameters every 10000 generations. The effective sample sizes were checked in Tracer v.1.6 and the acceptable values are higher than 200. The first 20% representing the burn-in phase were discarded and the remaining trees were combined in LogCombiner 1.10.2., summarized data and estimated in TreeAnnotator 1.10.2. Bars correspond to the 95% highest posterior density (HPD) intervals. The scale axis shows divergence times as millions of years ago (MYA).
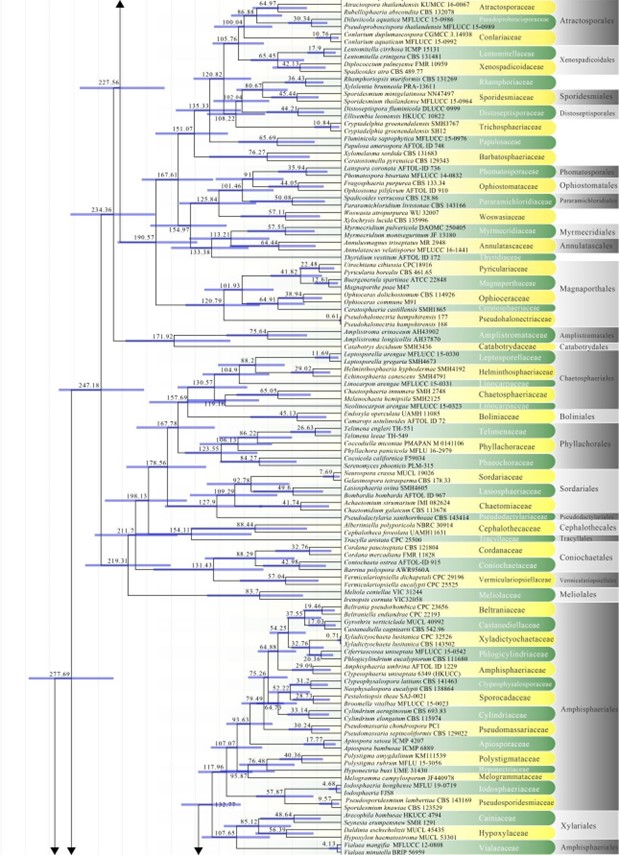
Figure 2 – Continued.
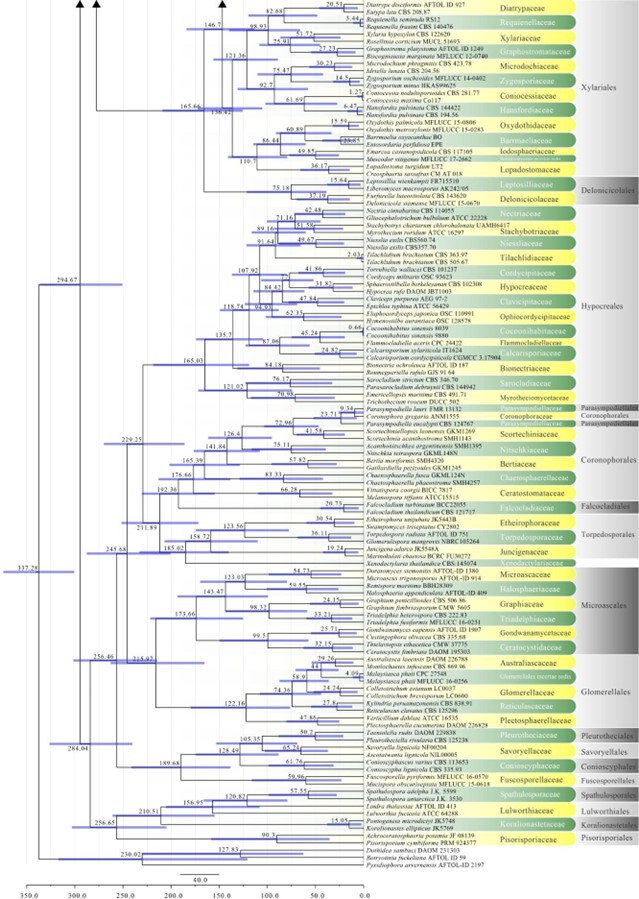
Figure 2 – Continued.
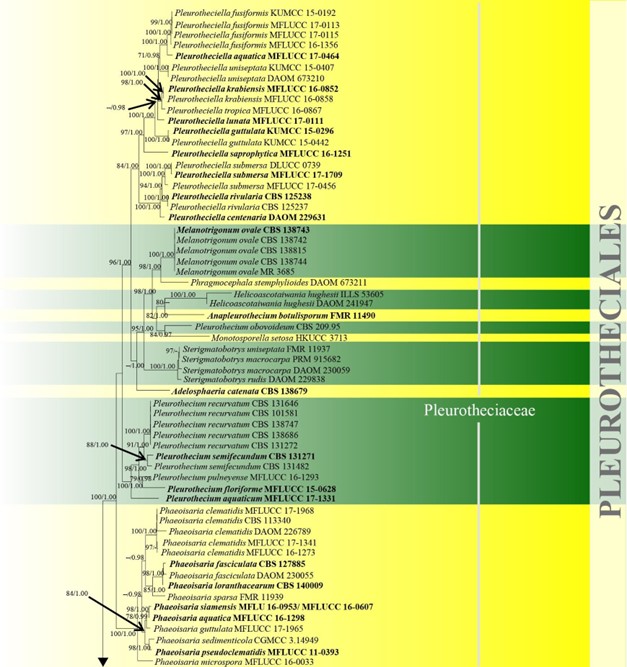
Figure 10 – Phylogram generated from maximum likelihood analysis based on combined LSU, SSU, ITS and rpb2 sequence data of Conioscyphales, Fuscosporellales, Parasympodiellales, Pleurotheciales and Savoryellales. One hundred and twenty-nine strains are included in the combined analyses which comprised 4253 characters (910 characters for LSU, 1499 characters for SSU, 759 characters for ITS and 1085 characters for rpb2) after alignment. Lindra thalassiae (AFTOL-ID 413) and Lulworthia uniseptata (AFTOL-ID 5014) are used as outgroup taxa. Single gene analyses and the phylogenies were similar in topology and clade stability. Tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best RaxML tree with a final likelihood value of -50774.805453 is presented. Estimated base frequencies were as follows: A = 0.233897, C = 0.263834, G = 0.294235, T = 0.208033; substitution rates AC = 1.342378, AG = 2.736022, AT = 1.339928, CG = 0.989078, CT = 6.301203, GT = 1.000000; gamma distribution shape parameter a = 0.440908. Bootstrap support values for ML greater than 70% and Bayesian posterior probabilities greater than 0.95 are given near the nodes. The tree is rooted with Lindra thalassiae (AFTOL-ID 413) and Lulworthia uniseptata (AFTOL-ID 5014). Type strains are in bold and black. The newly generated sequence is indicated in blue.
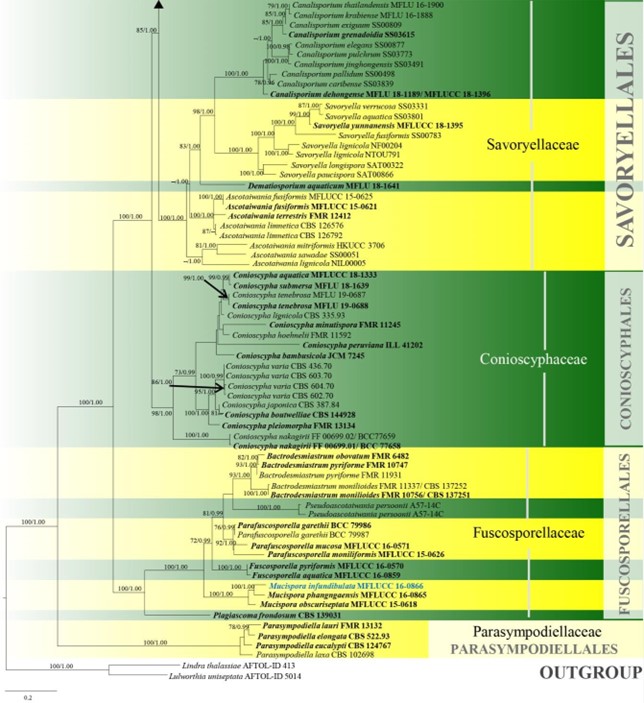
Figure 10 – Continued.
Families
