Noblesia Nakasone, gen. nov.
MycoBank number: MB 839627; Index Fungorum number: IF 839627; Facesoffungi number: FoF;
Basidiomes widely effused, up to 10 mm thick, soft ceraceous when fresh, drying crustaceous, hymenophore tuberculate, odontoid to hydnaceous, light yellow, bright orange-yellow or brown, turning red to black in 2% KOH solution, often developing deep cracks when dried, with or without hyphal cords; aculei or teeth single or fused at base, with red-dish brown bristles emerging from sides and apices; hyphal system monomitic with nodose-septate generative hyphae, agglutinated; single or fascicles of fusiform to cylindrical terminal hyphae covered with yellowish brown, mucilaginous matter or adherent crystalline material, projecting through hymenium and at aculeal apices (Fig. 4c); basidia narrowly clavate, basally clamped, 4-sterigmate; basidiospores ellipsoid to cylindrical, walls hyaline, slightly thickened, distinct, smooth, acyanophilous, not reacting in Mezler’s reagent.
Generic type: Noblesia crocea (Schwein.) Nakasone.
Etymology: For Mildred K. Nobles (1903–1993), Canadian mycologist who developed the “Species code” for the characterization and identification of wood-decay fungi in culture.
Remarks: Noblesia is a small genus characterized by yellow, orange, or brown basidiomes with a ceraceous texture that darken in KOH, fascicles of hyphae encased in yellowish brown mucilaginous material, narrowly clavate basidia, and small, ellipsoid basidiospores with hyaline, slightly thickened, smooth walls. Two species, with limited distribution and restricted host preferences, are accepted in the genus. Although P. fuscotuberculata was paired with P. hydnoidea (= N. crocea) in phylogenetic analyses by Huang and Zhao (2020, fig. 2), they are not considered congeneric since the former taxon lacks the brightly colored basidiomes and fasciculate hyphae encased by mucilaginous materials characteristic of both Noblesia species. Additionally, ITS sequences of P. fuscotuberculata are only 85% identical to P. hydnoidea (= N. crocea) as calculated from a BLAST® query (data not shown).Morphologically, Noblesia and Phlebia s.s. share ceraceous basidiomes, clamped generative hyphae, agglutinated subicular tissue, and clavate basidia. In contrast, cultures of N. crocea (as P. hydnoidea) grew very slowly on malt extract agar and gallic and tannic acid agars compared to the rapid growth by Phlebia s.s. (Nakasone 1990). Phylogenetic analyses place Noblesia in the Merulius clade, composed of morphologically diverse and distantly related taxa, that is distinct from the Phlebia clade (Fig. 3) and the Sarcodontia–Crustodontia lineage (Justo et al. 2017, fig. 5; Chen et al. 2018; Shen et al. 2018, fig. 2). Although Zmitrovich (2018) transferred P. hydnoidea to Merulius Fr., there is scant evidence that they are congeneric with respect to morphology and phylogeny. For example, effused to effused-reflexed, imbricate basidiomes with tomentose to hirsute abhymenial surface and pitted hymenophore of M. tremellosus, the generic type of Merulius, are unlike the effused, tuberculate to hydnaceous basidiomes in Noblesia. In addition, M. tremellosus lacks fasciculate hyphae (Fig. 4c) found in Noblesia, although they share monomitic hyphal systems with clamped generative hyphae that are coated with mucilaginous material, narrowly clavate basidia, and small, cylindrical to ellipsoid basidiospores. Finally, ITS sequences of M. tremellosus and P. hydnoidea are only 87% identical by BLAST® query, suggesting that they are not congeneric (data not shown). The generic status and placement of the other Phlebia species in the Merulius clade in Fig. 3 are unresolved and beyond the scope of our study. Justo et al. (2017, figs. 4–5) showed that species of Phlebia are distributed throughout the Meruliaceae based on phylogenetic analyses of three loci. Future phylogenetic studies must include more taxa and sequence data from additional loci to determine if Phlebia should be restricted to P. radiata and closely related species or expanded to encompass Aurantiopileus, Aurantiporus, Pappia, and other poroid taxa that are in the Phlebia clade.

Fig. 2 Radulomyces cope- landii basidiomes in situ. a LE–BIN–3899 from Georgia. b He20120923–21 from China;
R. licentii dried basidiomes. c SNU 9108160–56 from South Korea. d Dai 18173 from China. Scale bars in a, b, and d = 10 mm, c = 5 mm
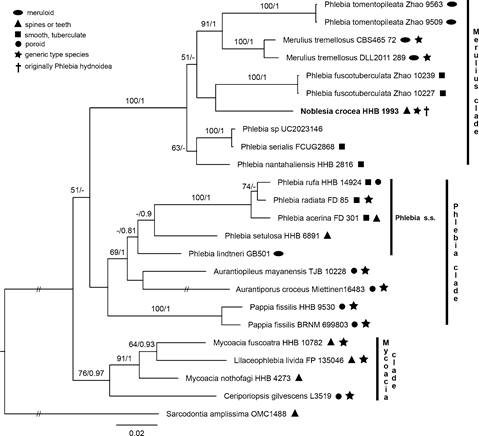
Fig. 3 Phylogeny of the Noblesia group inferred from BI analysis of ITS and nrLSU
sequences. Support values along branches are from ML Boot- strap (≥ 50%) and Bayesian pos- terior probabilities (PP ≥ 0.80), respectively

Fig. 4 Noblesia crocea. a HHB–10759 dried basidiome. b Close-up of aculei in HHB– 10759. b Fascicles of hyphae in aculei of PH00062606; Hydnum amplissimum. d Isolectotype, Sprague 297, UVMVT302881.Scale bars in a and b = 1 mm, c = 50 μm, d = 10 mm
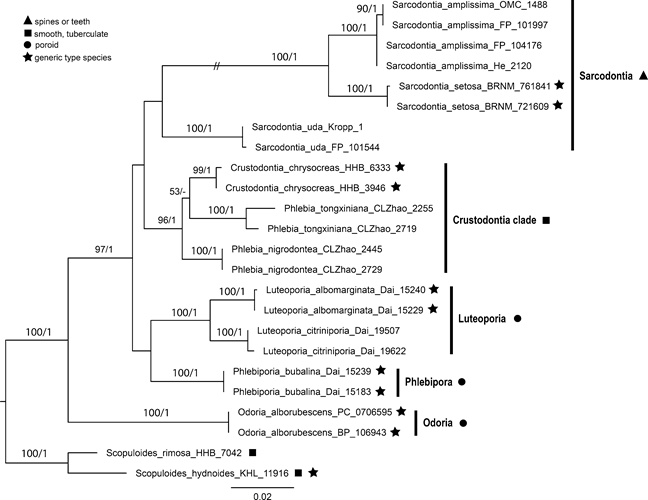
Fig. 5 Phylogeny of the Sarcodontia group inferred from ML analysis of ITS and nrLSU sequences. Support values along branches are from ML Bootstrap (≥ 50%) and Bayesian posterior probabilities (PP ≥ 0.80), respectively
Species
