Neodermea W.J. Li, D.J. Bhat & K.D. Hyde, gen. nov.
Index Fungorum number: IF557151, Facesoffungi number: FoF 07471
Etymology: Named after its morphology similar to Dermea.
Leotiomycetes, Leotiomycetidae, Medeolariales, Dermateaceae
Saprobic on plant host. Sexual morph: Apothecia black or drak brown, solitary or gregarious, erumpent, circular or undulate, sessile, narrowed below, leathery to horny in consistency, becoming more fleshy-leathery when moist. Hymenium at first concave, becoming plane or slightly convex, the margin at first thick, raised, later almost disappearing. Paraphyses hyaline, filiform, branched, septate, apex slightly swollen and glued together forming a yellowish epithecium. Tissue of hypothecium compact, pseudoparenchymatous, brownish. Asci 8-spored, cylindrical to clavate, short, stalked. Ascospores hyaline when young, becoming yellowish at maturity, uniseriate to biseriate, oblong-ellipsoid to ellipsoid-fusiform, 1–3-septate, straight or slightly curved (adapted from Grove 1946). Asexual morph: Conidiomata dark brown to black, stomatic, pycnidial, solitary or gregarious, immersed to semi-immersed, erumpent, globose to subglobose, unilocular or multi-locular, thick-walled, ostiolate. Ostiole cylindrical, single, with a wide channel, centrally located. Conidiomata wall composed of hyaline to brown, thick-walled cells of textura angularis. Conidiophores arising from the innermost wall layer of conidiomata, hyaline, cylindrical, septate. Conidiogenous cells enteroblastic, phialdic, cylindrical, usually integrated, determinate, smooth.
Type species: Neodermea rossica W.J. Li, D.J. Bhat & K.D. Hyde, sp. nov.
Notes: Dermea acerina (Peck) Rehm was introduced by Peck (1878) as Tympanis acerina Peck based on the morphology of apothecia. Subsequently, Saccardo (1889) transferred D. acerina to Scleroderris (Fr.) Bonord., although it differs from Scleroderris species in oblong-ellipsoid to ellipsoid-fusiform ascospores (Grove 1946). Rehm (1912) transferred it toDermea based on characters of apothecia, asci and ascospores. However, D. acerina has oblong- ellipsoid conidia, which are similar in form to the conidia of Pezicula species, rather than D. cerasi (elongate-fusiform to subfiliform conidia), and this has resulted in confusion about the generic concept. Groves (1938, 1941) established the connection between D. cerasi and Naemosphaera acerina (Peck) Höhn., based on cultural studies. A phylogenetic tree for Dermateaceae based on multi-locus sequence data shows that D. acerina CBS 161.38 is distinct from D. cerasi and forms a separate clade with two new collections (Fig. 129). Neodermea is introduced as a new genus to accommodate N. acerina and N. rossica. Neodermea rossica is designated as the type species.
Distribution: Canada, Italy, USA (Grove 1946; Verkley et al. 2003, this study)
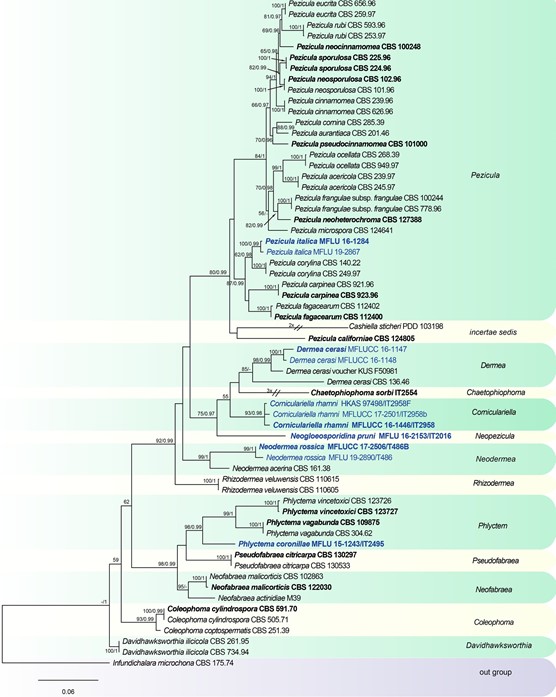
Fig. 129 Phylogenetic tree generated from a maximum likelihood analysis based on a concatenated alignment of LSU, ITS and rpb2 sequences data of Dermateaceae. The newly generated nucleotide sequences were compared against the GenBank database via blast search. Related sequences were obtained from (Chen et al. 2016) and GenBank. Sixty-two strains are included in the analyses, which comprise 2465 characters including gaps. Infundichalara microchona CBS 175.74 is used as the outgroup taxon. The tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best scoring RAxML tree with a final optimization likelihood value of − 15611.979066 is presented. The matrix had 938 distinct alignment patterns, with 16.00% of undetermined characters or gaps. Esti- mated base frequencies were: as follows A = 0.251579, C = 0.230991, G = 0.272796, T = 0.244634; substitution rates AC = 1.650679, AG = 3.597104, AT = 0.907930, CG = 0.800954, CT = 8.324139, GT = 1.000000; gamma distribution shape parameter α = 0.201567. Maximum likelihood (MLBS) bootstrap support values higher than 50%, and Bayesian posterior probabilities ≥ 0.95 (PP) are shown above or below the nodes. Hyphen (“–”) indicates a value lower than 50% for MPBS and MLBS and a posterior probability lower than 0.95 for BYY. The scale bar indicates 0.06 changes. Ex-type or ex- epitype strains are in bold. New isolates are in blue
Species
