Montagnula aquaticaY.R. Sun, Yong Wang bis & K.D. Hyde, sp. nov. (Fig.1)
MycoBank number: MB; Index Fungorum number: IF; Facesoffungi number: FoF 12922;
Holotype: xxx
Etymology: Referring to the aquatic habitat of the fungus.
Saprobic on submerged decaying wood in freshwater habitat. Sexual morph: Ascomata 250–430 μm long, 250–340 μm high, semi-immersed, solitary or scattered, globose, uniloculate, black, smooth-walled, with a central ostiole. Ostiole papillate, central. Peridium 10–22 μm wide, fused with host tissues, comprising of 2 layers of pale brown to brown cells of textura angularis. Hamathecium comprising 1.3–2.0 μm wide, numerous filamentous, branched, hyaline, septate, guttulate, pseudoparaphyses. Asci 110–130 × 13–19 μm ( = 122 × 15.5 μm, n = 10), bitunicate, 8-spored, cylindric-clavate, slightly curved. Ascospores 24–35 × 7.5–14 μm ( = 30.5 × 10.5 μm, n = 30), hyaline to yellow-brown when immature, dark brown when mature, 2- seriate, fusiform to broadly fusiform, 3-septate, widest at the centre, tapering towards ends, conical both end, guttulate, without appendages and mucilaginous sheath. Asexual morph: Not observed.
Culture characteristics: Ascospores germinating on PDA within 12 h at 25 ˚C, germ tubes produced from both ends. Colonies on PDA reaching 5 cm diam after 3 weeks at 25 ˚C, mycelium white, flossy, circular, slightly flattened, undulate, with the entire edge; white to yellow in reverse.
Material examined: Thailand, Chiang Rai Province, Bandu District, on decaying wood, 6 March 2021, Y.R. Sun, 26 (MFLU 22–xxxx, holotype; ex-type living culture MFLUCC 22-xxxx).
Notes: Montagnula aquatica phylogenetically formed as a distinct clade. Morphologically, M. aquatica can be distinguished by larger ascospores from its related species in Clade 1 (Fig. 1) ( 24–35 × 7.5–14 μm in M. aquatica vs. 18–25 × 5–88 μm in M. camporesii vs. 18–23.5 × 6.5–9.5 μm in M. cirsii vs. 20–23 × 7–9 μm in M. scabiosae) (Hongsanan et al. 2015; Hyde et al. 2016; Hyde et al. 2020; Du et al. 2021). In addition, M. aquatica has thinner peridium than M. cirsii (10–22 μm vs. 41–58.5 μm), M. aquatica has larger asci than M. camporesii (110–130 × 13–19 μm vs. 80–120 × 10–15 μm)(Hyde et al. 2016; Hyde et al. 2020). The results of base pair differences (Table 3) also support the establishment of M. aquatica as a new species (Jeewon and Hyde 2016). Thus, M. aquatica sp.nov is introduced and it is the first Montagnula species reported from freshwater habitats.
Table 3. Number of polymorphic nucleotide differences between M. aquatica with M. camporesii, M. cirsii and M. scabiosae (without gap).
| Species | Strain | ITS | LSU | SSU |
| M. camporesii | MFLUCC 16-1369 | 14(472bp) | 7(801bp) | 4(949bp) |
| M. cirsii | MFLUCC 13-0680 | 14(458bp) | 7(808bp) | 2(816bp) |
| M. scabiosae | MFLUCC 14-0954 | 15(446bp) | 7(802bp) | 6(964bp) |
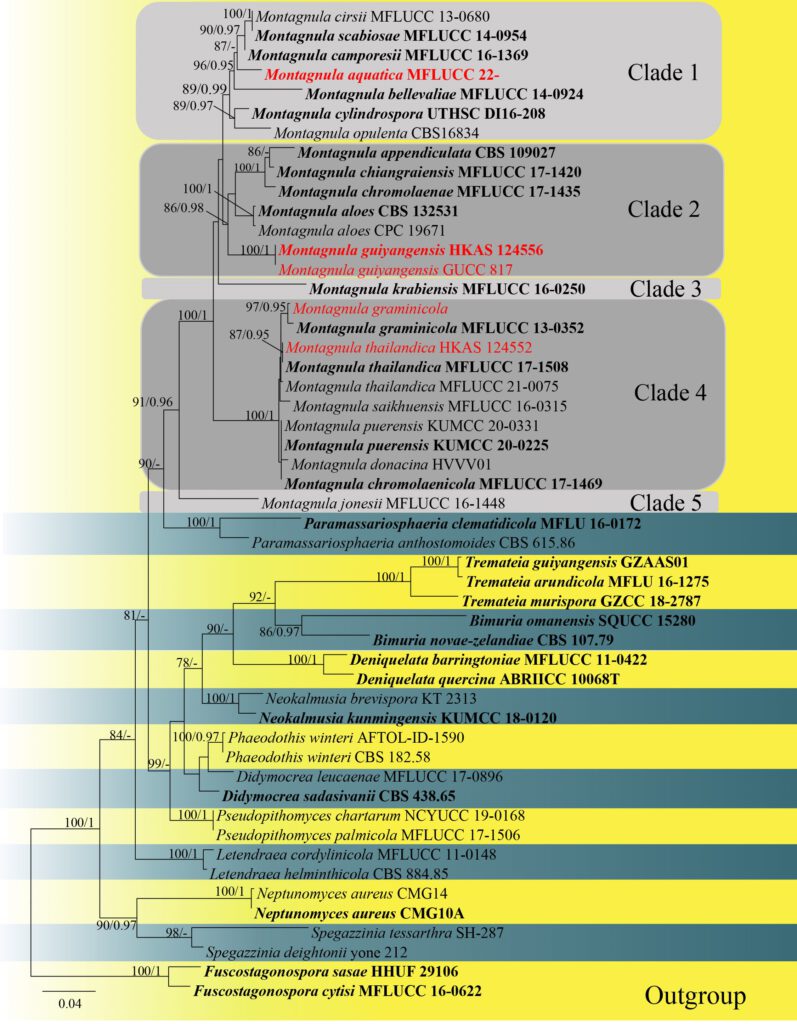
Figure 1. Maximum likelihood (RAxML) tree, based on analysis of a combined dataset of LSU, ITS and SSU sequence data. The tree is rooted with Fuscostagonospora sasae (HHUF 29106) and F. cytisi (MFLUCC 16-0622). Bootstrap support values for ML greater than 75% and Bayesian posterior probabilities greater than 0.95 are given near nodes, respectively. Ex-type strains are in bold, the new isolates are in red.
