Longinectria lagenoides O. Savary, M. Coton, E. Coton and J-L. Jany, sp. nov. Figs 3, 5
MycoBank number: MB 840950; Index Fungorum number: IF 840950; Facesoffungi number: FoF;
Etymology – From latin lagoena = bottle, refers to the observed phialide shape.
Holotype – UBOCC-A-120039, CBS 147588
Macromorphology – Based on UBOCC-A-120039 (CBS 147588), colony growth for 16 days at 25°C, colony surface on PDA was heterogeneously colored (Fig. 5A). The center was mainly brown (RGB [139,112,93]), the smooth margin was brown (RGB [104,95,78]) and intensified during growth. The intermediate zone appeared grey (RGB [164,167,173]) with brown shades because of the aerial white hyphae with powdery structures. Brown pigments were diffused in the agar around the colony. Colonies were radially furrowed with a convex center. Colony reverse was also heterogeneously colored (Fig. 5B), the center being brown (RGB [98,61,43]), then progressively clearer (RGB [128,90,51]) and highly visible on the reverse face, the fungal colony dug into the agar. Concerning fungal growth on M2Lev, colonies were beige with shades of brown and, in particular, in the regular margins and convex center (Fig. 3). Very aerial hyphae were observed with powdery and/or cottony structures and the mycelium dug into agar.
Micromorphology – Ascomatal state unknown. Lateral phialides with different sizes were observed (on average 44 µm, 15–72 µm), some being very long (size 153–237 µm) (Fig. 5C–D) while others are short and could even be assimilated to phialidic peg. Macroconidia 0–3 septate, very straight in shape with an apical form, blunt to papillate, and basal cell very pronounced and notched (Fig. 5E –5J). Their size is on average 17 x 4 µm (12–29 x 3–7 µm) length range. Microconidia 0–1 septate ovoid to allantoid shape (8–12 x 3–5), apical form blunt and basal cell slightly notched (Fig. 5E, G). After several weeks of growth, globoses chlamydospores were observed typically intercalary, or terminal with mainly ≥ 2 chlamydospores. SNA medium did not provide any supplementary information.
Growth characteristics on PDA – Slow growth was observed at 25°C, colony diameter measuring 53.0 ± 1.6 mm versus 32.3 ± 0.5 mm at 15°C and the growth rates were estimated at 3.7 ± 0.1 mm/days versus 2.3 ± 0.0 mm/days, respectively. Moreover, L. lagenoides growth was observed between 5°C and 25°C, colony diameters measuring at 14 days 4.5 ± 0.0 mm at 5°C, 13.0 ± 0.0 mm at 10°C, 44.5 ± 2.1 mm at 20°C, and no growth at 30°C and 37°C.
Secondary metabolites – No known mycotoxins already described to be produced by Fusarium, Penicillium, Aspergillus or Alternaria spp. were detected. For most detected compounds, except ergosterol, extrolites could not be identified but belonged to 38 chromophore families (Table 3). These secondary metabolites again included the “emon” chromophore or corresponded to extra apolar free fatty acids (including linoleic acid and probably oleic acid), mid-cyclic lipopeptides with tyrosine in the cyclic peptide, indole alkaloids, 2-pyruvoylaminobenzamide-like molecules but also an alkylphenone chromophore and atrovenetin chromophore (Table 3).
Substrate and distribution – To date, L. lagenoides has been solely isolated from European cheese.
Material examined – France, isolated from Swiss cheese, O. Savary, 22 Nov. 2018, holotype UBOCC-A-120039, CBS 147588.
Additional material examined – France, isolated from Swiss cheese, O. Savary, 20 Nov. 2018, UBOCC-A-120038; France, isolated from Swiss cheese, O. Savary, UBOCC-A-120041, 27 Nov. 2018; France, isolated from semi-hard Swiss cheese, J. Ropars, ESE 00140, 2018.
Note – Few macroscopically differences were observed among the four isolates observed on PDA (Fig. 3). Indeed, colony color was more or less pronounced (RGB [156,153,136] or [157,157,162]) depending on the considered strain), and in particular, one strain (UBOCC-A- 120038) remained yellow/beige (RGB [151, 153, 148]) even with natural light exposure during several days. Radial furrows with a convex center were mainly pronounced for UBOCC-A-120038 and UBOCC-A-120039 but also slightly more observable for ESE-00140. The colony reverse could be beige (RGB [140,134,121]) to brown (RGB [143,109,80]) depending on the strains.
Table 3 Production of different chromophore groups of secondary metabolites by Nectriaceae spp.
| Chromophore family | Identity or chromophore | Bisifusarium allantoides | Longinectria lagenoides | Bisifusarium penicilloides | Longinectria verticilliforme |
| UBOCC-A-120036HT | UBOCC-A-120039HT | UBOCC-A-120021HT | UBOCC-A-120043HT | ||
| Alk 1-3 | indole alkaloid chromophore | + | + | + | – |
| Alk 4 | indole alkaloid chromophore | – | + | – | – |
| Aspham | alkylphenone chromophore | – | + | – | – |
| Atrov | atrovenetin chromophore | – | + | – | – |
| Cyt | tyrosine chromophore | + | + | + | + |
| e1-12 | no chromophore | + | + | + | + |
| e15-21 | no chromophore | + | + | + | + |
| e38-48 | fatty acid | + | – | + | – |
| Emon | + | + | + | + | |
| Ergosterol | (common membrane | + | + | + | + |
| constituent) | |||||
| Ffa 1-4 | fatty acid | + | + | + | + |
| Flit | – | + | – | – | |
| Fluorescing compound | – | + | – | – | |
| at retention time 12.95 | |||||
| Linoleic acid | linoleic acid | + | + | + | + |
| Pal | – | – | + | – | |
| Pyru | pyruvoylaminobenzamide | + | + | + | – |
| chromophore | |||||
| RED | – | – | – | + | |
| Snor | + | – | + | – | |
| Ulm | + | – | + | + | |
| Vit | + | + | + | + | |
| Vor | + | + | + | + | |
| xx | + | – | – | + |
The production and detection by HPLC-DAD of chromophore family is highlighted by the “+” symbol and its absence is represented by the “-” symbol
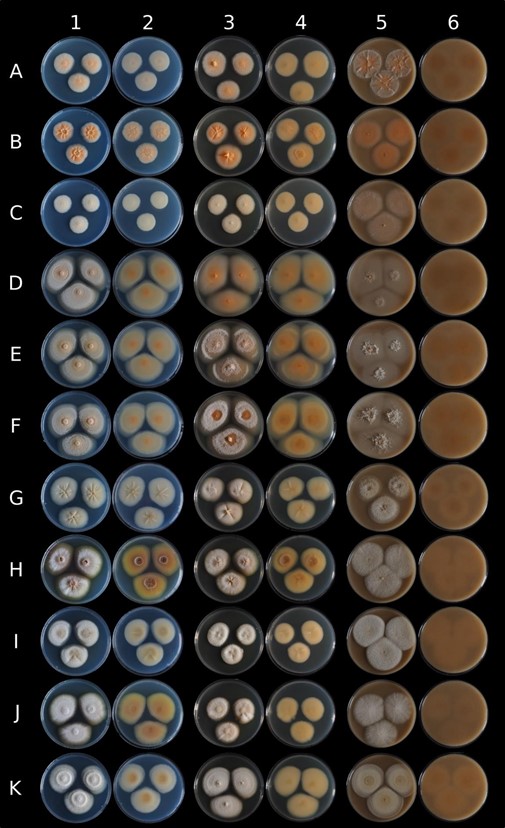
Figure 3 – Macroscopic comparison of eleven strains belonging to four new Nectriaceae species on different culture media. Lines A to K respectively correspond to Bisifusarium penicilloides VTT-D- 041022, B. penicilloides UBOCC-A-120034, B. penicilloides UBOCC-A-120021, Bisifusarium allantoides UBOCC-A-120035, B. allantoides UBOCC-A-120036, B. allantoides UBOCC-A- 120037, Longinectria lagenoides UBOCC-A-120038, L. lagenoides UBOCC-A-120039, L. lagenoides UBOCC-A-120041, L. lagenoides ESE-00140 and Longinectria verticilliforme UBOCC-A-120043 grown during 14 days at 25°C on Potatoes Dextrose Agar (column 1 & 2: observe & reverse), M2Lev (column 3 & 4: observe & reverse) and oatmeal agar (column 5 & 6: observe & reverse).
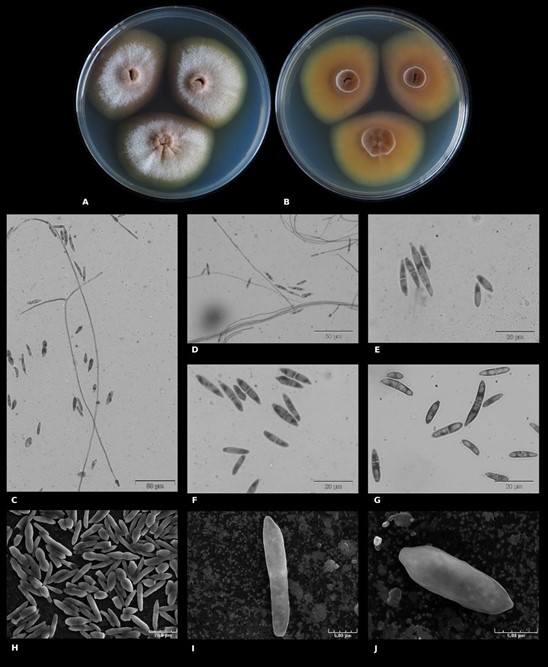
Figure 5 – Macroscopic morphological and microscopic characteristics of Longinectria lagenoides (UBOCC-A-120039HT). A Colony on PDA (14 days at 25°C). B Colony reverse on PDA (14 days at 25°C). C–D phialides. E–J micro- and macro- conidia. C–G microscopic acquisitions using classical microscopy, H–J: microscopic acquisition using scanning electron microscopy.
