Kaarikia abrahamsonii C. Mayers, T.C. Harr. & Roeper, sp. nov. FIG. 7
MycoBank number: MB 824947; Index Fungorum number: IF 824947; Facesoffungi number: FoF14484;
Typification: USA. MICHIGAN: Isabella County, Chippewa Township, ambrosia growth in galleries of X. politus in Betula papyrifera, Jun 2014, R. Roeper M599 (holotype BPI 910623). Ex-type culture CBS 144155 (= A1264; dried culture BPI 910624).
Etymology: After Lawrence P. Abrahamson (SUNY- ESF), who performed pioneering work on the mycangia and ambrosia fungi of Xyloterinus politus.
Colonies on malt yeast extract agar 45–56 mm diam after 7 d at 25 C, odor earthy, surface growth superficial, umber with irregular patches of buff to mouse gray aerial hyphae, margin irregular, submerged, dendroid, buff, becoming dense and ochreous, reverse umber to olivaceous black, becoming chestnut. Chlamydospores formed in culture hyaline to red-brown, thick-walled, single-celled or rarely septate, globose to irregular, 6.5–22.5 × 8.0–28 µm, borne terminally or intercalary inside red-brown hyphae, separating by tearing of the pigmented hyphal wall. Gallery growth black, superficial in main tunnels; white to green- gray in larval cradles and egg niches, with thick mat com- posed of palisades of conidiophores. Conidiophores simple, unbranched, erect, septate or aseptate, bearing single terminal conidia. Conidia obovoid to pyriform, hyaline, thick- walled, 6–18 × 12.5–35.5 µm, truncate, aseptate or rarely 1- or 2-septate. Chlamydospores in gallery as in culture but globose, smaller, 6.0–13.5 µm diam. Mycangium growth spherical, thick-walled, 10–17.5 µm diam.
Geographic distribution: Known directly from Michigan; presumably shares the larger reported range of its host, X. politus, whose distribution spans the northern Midwest and northeastern USA (MacLean and Giese 1967).
Known habitat: In sapwood galleries and prothoracic mycangia of the ambrosia beetle X. politus, which makes its galleries in a variety of hardwoods (MacLean and Giese 1967).
Notes: The prothoracic ambrosia fungus of X. politus is unique and appears to be unrelated to other known mycangial mutualists of ambrosia beetles. In galleries of X. politus, it dominates larval cradles with its club- shaped conidia and tunnels with its darkly pigmented hyphae, as illustrated by MacLean and Giese (1968), Abrahamson (1969), and Abrahamson and Norris (1969). A culture of Abrahamson and Norris’ UWE- 132M, which we presume to be K. abrahamsonii, was maintained by the University of Wisconsin–Madison Department of Entomology (Abrahamson and Norris 1969) and has since been lost (Craig Brabant, interim academic curator, pers. comm.), but color photographs of UWE-132M provided by L. Abrahamson resembled our isolates of K. abrahamsonii.
Other cultures examined: USA. MICHIGAN: Montcalm County, Alma College Ecological Tract, from prothorax of female X. politus in Populus sp., 21 May 2014, R. Roeper A1268 (CBS 142646; dried culture BPI 910625); Isabella County, Chippewa Township, from gallery of X. politus in Betula papyrifera, Jun 2014, R. Roeper A1262; from gallery of X. politus in B. papyrifera, Jun 2014, R. Roeper A1263; from prothorax of female X. politus in B. papyrifera, Jun 2014, R. Roeper, four cultures from separate females: A1265, A1266, A1267, A1269.
Other specimen examined: USA. MICHIGAN: Montcalm County, Alma College Ecological Tract, galleries of X. politus in Acer rubrum, 20 Aug 2013, R. Roeper M598 (BPI 910626).
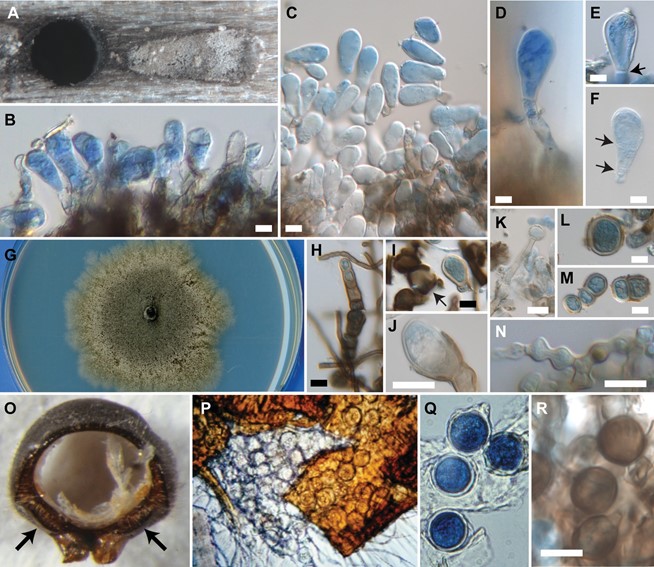
Figure 7. Kaarikia abrahamsonii and prothoracic mycangia of Xyloterinus politus. A–F. Conidia and conidiophores in galleries. A. Larval cradle with ambrosia growth next to main tunnel. B. Palisade of conidiophores in larval cradle. C. Detached conidia in egg niche. D. Simple conidiophore. E. Terminal conidium with truncate attachment (black arrow). F. Detached conidium with two septa (black arrows). G–N. Culture morphology. G. Growth at 7 d on MYEA. H–M. Chlamydospores. H. Terminal and intercalary chlamydospores, breaking free. I. Free chlamydospore on right, with empty-pigmented sheath (black arrow) on left. J. Terminal, with pigmented outer sheath visible. K. Terminal on simple hypha. L. Solitary chlamydospore. M. Chained chlamydospores. N. Beaded hyphae in culture. O–Q. Prothoracic mycangium and mycangial propagules. O. Posterior aspect of X. politus prothorax with rest of body removed, showing paired prothoracic basin mycangia (black arrows). P. Prothoracic basin mycangium cracked under coverslip, with mycangial spores inside. Q. Spherical mycangial propagules. R. Spherical chlamydospores at cradle entrance. A, B, D, F, R. In Acer rubrum (BPI 910626). C. In Betulae papyrifera (BPI 910623; holotype). G–N. Isolate A1268 (CBS 142646). A, O. By stereo microscopy. B–F, H–N, R. By Nomarski interference microscopy of material stained with cotton blue. P, Q. By light microscopy. P. Unstained. Q. Stained with Trypan blue. G. With Epson 10000XL scanner, plate diam = 90 mm. Bars = 10 μm.
