Diaporthomycetidae Senan., Maharachch. & K.D. Hyde, Fungal Divers. 72: 208 (2015)
MycoBank number: MB 551051; Index Fungorum number: IF 551051; Facesoffungi number: FoF 00594;
The subclass Diaporthomycetidae was introduced by Maharachchikumbura et al. (2015) for some taxa already placed in Sordariomycetidae, but that were phylogenetically and morphologically distinct from Sordariomycetidae. Members of Diaporthomycetidae occur in both aquatic and terrestrial habitats as saprobes, pathogens, or endophytes. Previously there were ten orders in this subclass (Hongsanan et al. 2017). Crous et al. (2017a) introduced Pararamichloridiales and Crous et al. (2019a) introduced Sporidesmiales. Hyde et al. (2017a) proposed Catabotryales based on evolutionary data and here we formally introduce it. Currently there are 15 orders and 65 families in this subclass (Hyde et al. 2017a, this paper). The divergence time for Diaporthomycetidae is estimated as 247 MYA (Fig. 2). The orders and families in this subclass are mostly well-supported in our phylogenetic analysis (Figs 6, 8, 13, 14, 18).
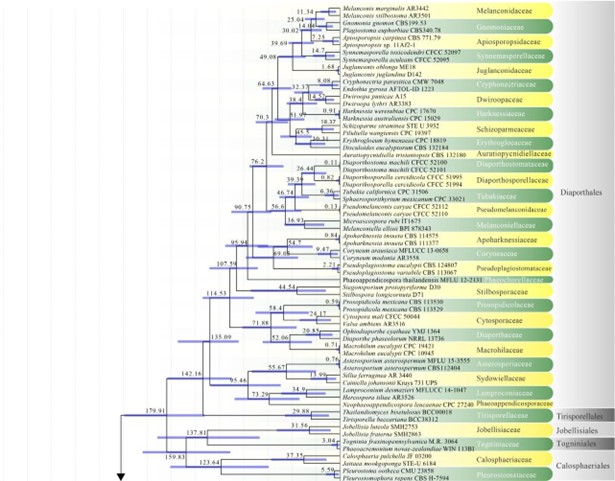
Figure 2 – The maximum clade credibility (MCC) tree, using the same dataset from Fig. 1. This analysis was performed in BEAST v1.10.2. The crown age of Sordariomycetes was set with Normal distribution, mean = 250, SD = 30, with 97.5% of CI = 308.8 MYA, and crown age of Dothideomycetes with Normal distribution mean = 360, SD = 20, with 97.5% of CI = 399 MYA. The substitution models were selected based on jModeltest2.1.1; GTR+I+G for LSU, rpb2 and SSU, and TrN+I+G for tef1 (the model TrN is not available in BEAUti 1.10.2, thus we used TN93). Lognormal distribution of rates was used during the analyses with uncorrelated relaxed clock model. The Yule process tree prior was used to model the speciation of nodes in the topology with a randomly generated starting tree. The analyses were performed for 100 million generations, with sampling parameters every 10000 generations. The effective sample sizes were checked in Tracer v.1.6 and the acceptable values are higher than 200. The first 20% representing the burn-in phase were discarded and the remaining trees were combined in LogCombiner 1.10.2., summarized data and estimated in TreeAnnotator 1.10.2. Bars correspond to the 95% highest posterior density (HPD) intervals. The scale axis shows divergence times as millions of years ago (MYA).
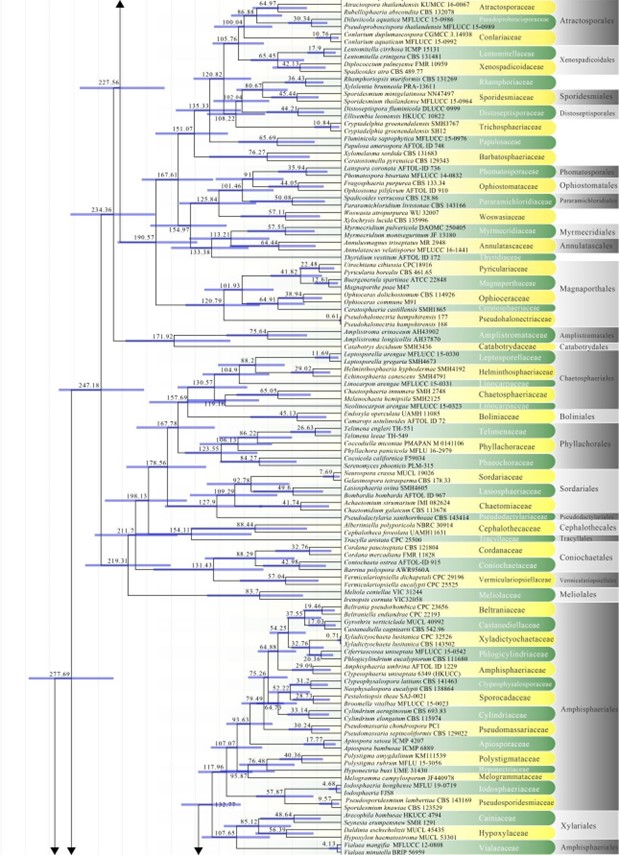
Figure 2 – Continued.
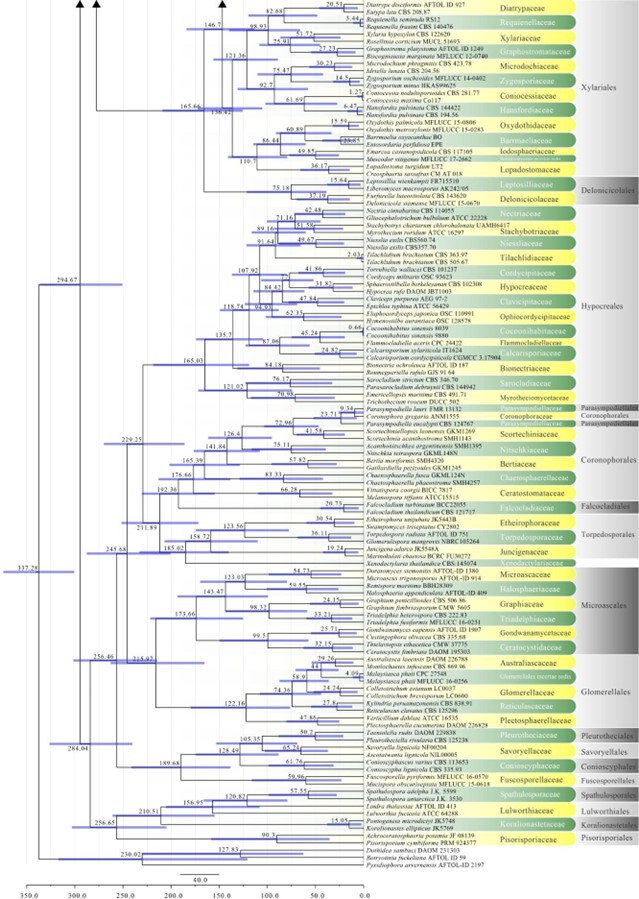
Figure 2 – Continued.
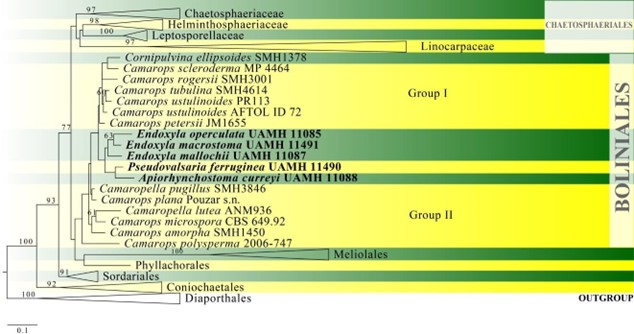
Figure 6 – Phylogram generated from maximum likelihood analysis based on combined LSU, ITS and tub2 sequence data of Boliniales. Related sequences are taken from Læssøe et al. (2013). Seventy-three strains are included in the combined analyses which comprised 2081 characters (867 characters for LSU, 561 characters for ITS, 653 characters for tub2) after alignment. Members of Diaporthales are used as outgroup taxa. Single gene analyses were also carried out and the phylogenies were similar in topology and clade stability. The best RAxML tree with a final likelihood value of -34809.341208 is presented. Estimated base frequencies were as follows: A = 0.209463, C = 0.298053, G = 0.289389, T = 0.203095; substitution rates AC = 1.086242, AG = 2.378803, AT = 1.369068, CG = 1.213916, CT = 6.024342, GT = 1.000000; gamma distribution shape parameter a = 0.567627. Bootstrap support values for ML greater than 60% are given near the nodes. Ex-type strains are in bold. The newly generated sequences are indicated in blue.

Figure 8 – Phylogram generated from maximum likelihood analysis based on combined LSU and ITS sequence data of Chaetosphaeriales and Tracyllalales taxa. Ninety-six strains are included in the combined analyses which comprised 1695 characters (1081 characters for LSU, 614 characters for ITS) after alignment. Neurospora crassa MUCL 19026 and Gelasinospora tetrasperma CBS 178.33 (Sordariaceae, Sordariales) are used as outgroup taxa. Single gene analyses were carried out and the phylogenies were similar in topology and clade stability. Tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best RaxML tree with a final likelihood value of – 23777.689886 is presented. Estimated base frequencies were as follows: A = 0.231060, C = 0.264793, G = 0.308265, T = 0.195882; substitution rates AC = 1.388486, AG = 1.836207, AT = 1.649563, CG = 0.971659, CT = 6.316962, GT = 1.000000; gamma distribution shape parameter a = 0.460297. Bootstrap support values for ML greater than 75% and Bayesian posterior probabilities greater than 0.95 are given near the nodes. Ex-type strains are in bold. The newly generated sequences are indicated in blue.
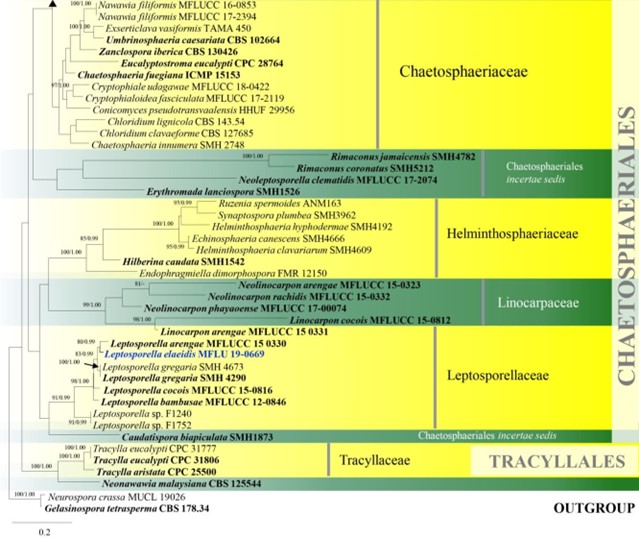
Figure 8 – Continued.
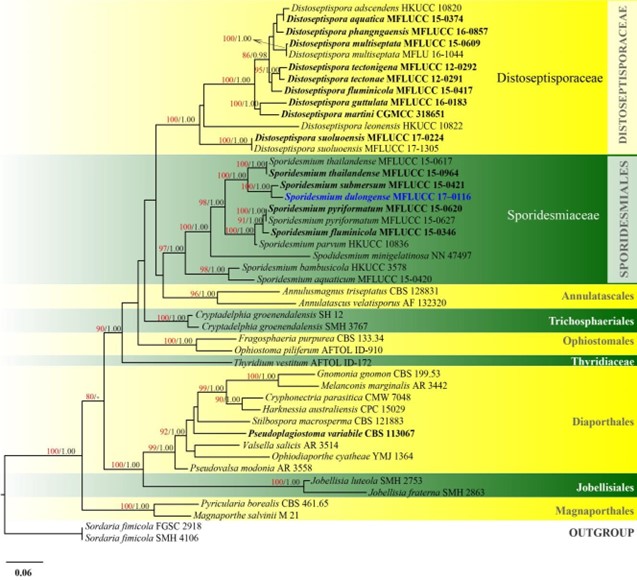
Figure 13 – Phylogram generated from maximum likelihood analysis based on combined ITS, LSU, rpb2 and tef1 sequence data of Distoseptisporales and Sporidesmiales. Forty-six strains are included in the combined analyses which comprised 3153 characters (789 characters for LSU, 546 characters for ITS, 1038 characters for rpb2, 779 characters for tef1) after alignment. Single gene analyses were carried out and the phylogenies were similar in topology and clade stability. Tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. The best RaxML tree with a final likelihood value of -22582.382236 is presented. Estimated base frequencies were as follows: A = 0.236961, C = 0.261709, G = 0.291323, T = 0.210007; substitution rates AC = 1.206796, AG = 2.260744, AT = 1.272906, CG = 1.049687, CT = 6.479176, GT = 1.000000; gamma distribution shape parameter a = 0.651156. Bootstrap support values for ML greater than 75% and Bayesian posterior probabilities greater than 0.95 are given near the nodes. The tree is rooted with Sordaria fimicola (FGSC 2918 and SMH 4106). Ex-type strains are in bold. The newly generated sequences are indicated in blue.
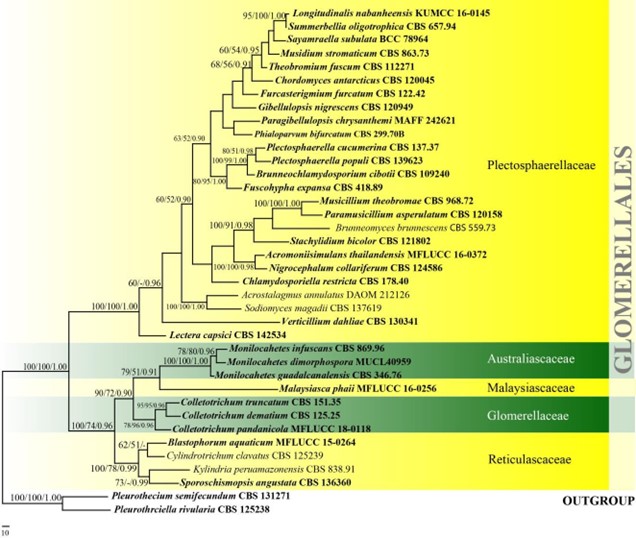
Figure 14 – One of the 100 most phylogenetic tree generated by maximum parsimony analysis of combined LSU, ITS and tef1 sequence data of species in Glomerellales. Thirty-eight strains are included in the analyses, which comprise 2162 characters including gaps (800 characters for LSU, 558 characters for ITS, 793 characters for tef1) after alignment. Pleurothecium semifecundum (CBS 131271) and Pleurotheciella rivularia (CBS 125238) (Pleurotheciaceae, Pleurotheciales) are used as outgroup taxa and the tree is rooted with. Single gene analyses were carried out and the phylogenies were similar in topology and clade stability. Tree topology of the maximum parsimony analysis is similar to the maximum likelihood and Bayesian analysis. The maximum parsimonious dataset consisted of 1189 constant, 630 parsimony-informative and 343 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum often equally most parsimonious trees with a length of 2672 steps (CI=0.522, RI=0.666, RC=0.348, HI=0.478) in the first tree. Bootstrap support values for MP and ML greater than 50% and Bayesian posterior probabilities greater than 0.90 are given near the nodes. Ex-type strains are in bold. The newly generated sequences are indicated in blue.
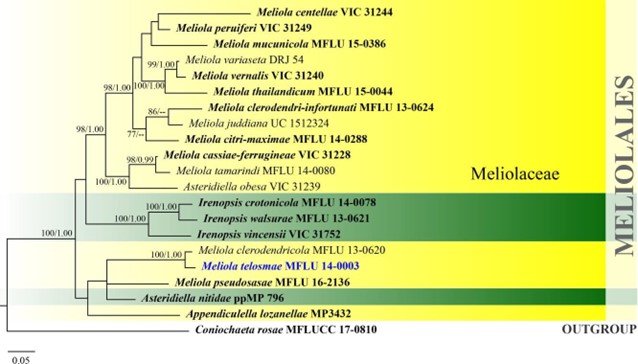
Figure 18 – Phylogram generated from Bayesian inference based on combined LSU, SSU and ITS sequence data of Meliolales. Twenty-one strains are included in the combined analyses which comprised 2688 characters (869 characters for LSU, 1020 characters for SSU, 799 characters for ITS) after alignment. Coniochaeta rosae (MFLUCC 17-0810) is used as outgroup taxa. Single gene analyses were carried out and the phylogenies were similar in topology and clade stability. Tree topology of the maximum likelihood analysis is similar to the Bayesian analysis. Estimated base frequencies were as follows: A = 0.25335, C = 0.2223, G = 0.281553, T = 0.242798; substitution rates AC = 1.021441, AG = 3.556619, AT = 2.123549, CG = 0.380727, CT = 6.542902, GT = 1.000000; gamma distribution shape parameter α = 0.549005. Bootstrap support values for ML greater than 75% and Bayesian posterior probabilities greater than 0.95 are given near the nodes. Ex-type strains are in bold. The newly generated sequences are indicated in blue.
