Cinnabaria boliviana Wilk & Lücking, sp. nov. FIG. 5
MycoBank number: MB 836185; Index Fungorum number: IF 836185; Facesoffungi number: FoF;
Typification: BOLIVIA. DEPT. COCHABAMBA: Prov. Quillacollo, East Cordillera, area of Inkarraya- Sipesipe, dry inter-Andean valleys, 17°29′25″S, 66°22′ 09″W, 3146 m, rocky and shrubby slope, sunny place, E exposition, 17 Dec 2004, Wilk 3221a (holotype KRAM-L-71757, isotype LPB).
Diagnosis: Thallus saxicolous, areolate, sublobate at margin, pale yellow orange. Apothecia abundant, immersed, red, contrasting against the thallus. Ascospores polarilocular, medium-sized with thin septum, 11.9 × 6.4 μm, septum 2.6 μm. Pycnidia ±comple- tely immersed. Colorless crystals present inside of the thallus and apothecia.
Etymology: The epithet refers to the occurrence of the species in Bolivia.
Thallus 200‒250 µm thick, areolate, sublobate at margin, irregular in outline, tightly attached to substratum, ca. 2 cm wide, pale yellow orange, K+ purple, pruinose in central part, some pseudocyphellae present, areoles 0.3‒0.7 mm wide, polygonal, slightly convex, marginal sublobes 0.3‒1.4 mm long, 0.2‒0.9 mm wide at the tips, slightly convex, even or widened; vegetative propagules absent; prothallus absent; cortex thick, 40‒85 µm, para- plectenchymatous, uneven, distinct cortex cones present, necral layer present, 8.5‒20.0 µm thick, anthraquinone pigments present, colorless crystals usually present; algal layer discontinuous, algae in dis- tinct groups; medulla completely filled by colorless crystals, which going to the cortex by fungal stocks; photobiont trebouxioid, cell globose, 6‒14 µm diam.
Apothecia abundant, scattered, round to angular, immersed, sometimes slightly emergent, zeorine or almost lecanorine, 0.2‒1.1 mm wide; disc first concave then plane, red, K+ purple, contrasting against thallus, epruinose, slightly cracked; apothecial margin level with disc, concolorous with thallus. Parathecium thin, ca. 75 µm, prosoplectenchymatous, anthraquinone pigments present, colorless crystals may be present as thin layer along the parathecium; amphithecium thin to thick, 50‒140 µm, algae abundant, forming discontinuous layer, cortex inconspicuous, paraplectenchymatous, anthraquinone pigments present, colorless crystals may be present; colorless crystals may be present in medulla of apothecia; epihymenium reddish yellow, granular (anthraquinones), K+ purple; hymenium 70 µm thick; hypothecium 160‒170 µm thick, prospoplectenchymatous, hyaline with yellowish tinge, K−, oil droplets abundant, stipe present with some colorless crystals; paraphyses simple to branched, with upper cell up to 5 µm wide; asci 8-spored; ascospores polarilocular, ellip-soid, hyaline, thin-walled, (10.0‒)11.9 ± 1.0(‒15.0) × (5.0‒) 6.4 ± 0.4(–7.5) μm, length/width ratio ca. 1.9, ascospore septa (2.0‒)2.6 ± 0.5(‒3.5) μm, length/septa width ratio ca. 4.7 (n = 49). Pycnidia abundant, ostiole orange to red, ±immersed, indistinct, conidia bacilliform, 2‒4.5 × 1 µm.
Habitat and distribution: Cinnabaria boliviana grows in dry inter-Andean valleys, on calcareous rocks together with Wetmoreana sp. 1, in well-lit conditions, at an altitude of ca. 3000 m. Known only from Bolivia.
Paratypes: BOLIVIA. DEPT. COCHABAMBA: Prov. Quillacollo, East Cordillera, area of Inkarraya-Sipesipe, dry inter-Andean valleys, 17°29′25″S, 66°22′09″W, 3146 m, E exposition, rocky and shrubby slope, sunny place, 17 Dec 2004, Wilk 3222 (LPB); 17°28′39″S, 66°21′43″W, 2846 m, NE exposition, 17 Dec 2004, Wilk 3292 (B).
Remarks: The species is characterized by a yellowish, areolate thallus, sublobate at the margin, and immersed, red apothecia, contrasting with the thallus color. The taxon is most similar to species of the “Caloplaca” cinnabarina group, especially “Caloplaca” montisfracti S.Y. Kondr. & Kärnefelt and “Caloplaca” rubelliana (Ach.) Lojka (Wetmore and Kärnefelt 1999; Kondratyuk et al. 2007). The new genus Brownliella was proposed for this taxonomic group (Kondratyuk et al. 2013) but was later split into Brownliella s.s. and Neobrownliella (Kondratyuk et al. 2015a; see also comments in TABLE 2); in addition, “Caloplaca” cinnabarina does not appear to be a member of Brownliella as suggested by Kondratyuk et al. (2013) but clusters separately as unsupported sister to Filsoniana in our tree (FIG. 2). All these lineages are genetically separate from the new species, since they cluster near Group III in our phylogenetic tree (FIG. 2). In general, Cinnabaria boliviana differs from the members of the “Caloplaca” cinnabarina group by having a yellowish and distinctly larger thallus, with thicker thalline cortex, and larger apothecia and ascospores (ascospores up to 12 µm long in members of the C. cinnabarina group). “Caloplaca” rubelliana, which is most similar to Cinnabaria boliviana, differs from the latter in having a gray-orange to orange, thin thallus that lacks lobules at the margins, and a gray prothallus (Wetmore and Kärnefelt 1999). The Australian species Neobrownliella montisfracti differs from Cinnabaria boliviana in having a pinkish, continuous to areolate thallus, becoming very thin toward the thallus margin, and smaller apothecia and ascospores (Kondratyuk et al. 2007).
Caloplaca fernandeziana (Zahlbr.) Follmann & Redón is another species somewhat similar to Cinnabaria boliviana in producing red apothecia contrasting against a yellowish thallus, but it differs in having a thinner, usually discontinuous thallus, a distinct, black prothallus, and sessile, biatorine apothecia. Caloplaca fernandeziana appears to be a Chilean endemic (Zahlbruckner 1917; Malme 1926; Follmann and Redón 1972). It was considered to possibly belong to Teuvoahtiana in Xanthorioideae (Kondratyuk et al. 2017), but our analyses indicate that it is nested within Caloplacoideae, representing an undescribed generic lineage (FIG. 1, TABLE 2).
Figure 1. Teloschistaceae phylogeny. Topology derived from RAxML analysis performed on a combined ITS, 28S, and mtSSU super- matrix for 169 terminals. Amandinea punctata was used to root the tree. The two support values associated with branches indicate Bayesian posterior probabilities estimated with MrBayes and maximum likelihood bootstrap values from RAxML. Only posterior probabilities ≥0.95 or bootstrap values ≥70% are indicated. Thickened branches denote significant support both for posterior probabilities (≥0.95) and bootstrap values (≥70%). Black arrows indicate newly obtained sequences.
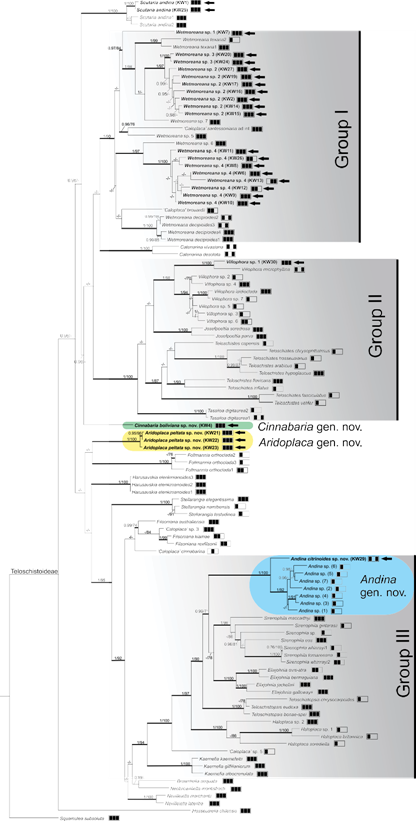
Figure 2. Teloschistoideae phylogeny. Topology derived from a RAxML analysis performed on a combined ITS, 28S, and mtSSU supermatrix for 111 terminals. Squamulea subsoluta was used to root the tree. The two support values associated with branches indicate Bayesian posterior probabilities estimated with MrBayes and maximum likelihood bootstrap values from RAxML. Only posterior probabilities ≥0.95 or bootstrap values ≥70% are indicated. Thickened branches denote significant support both for posterior probabilities (≥0.95) and bootstrap values (≥70%). The 3-box grids indicate the loci available for those particular specimens (ITS, 28S, and mtSSU, respectively) used in both BI and ML analyses. Black arrows indicated newly obtained sequences.
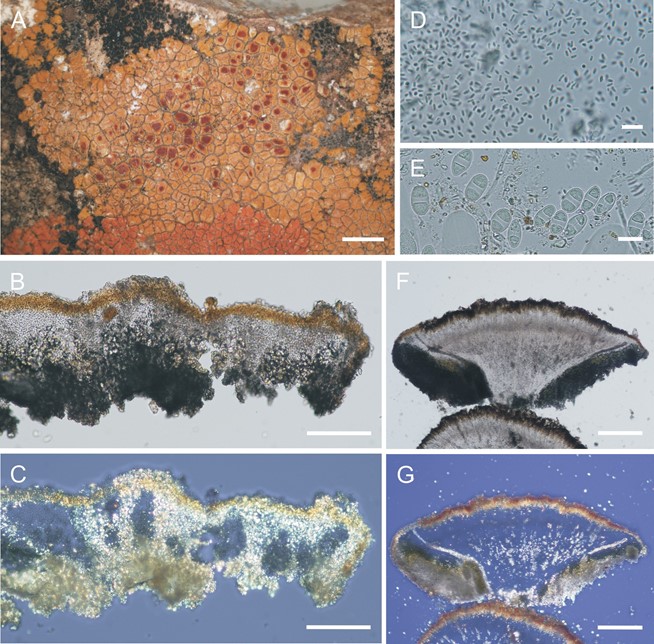
Figure 5. Cinnabaria boliviana (Wilk 3221a, holotype). A. Habit of thallus. B, C. Cross-section of thallus in regular (B) and polarized light (C). D. Pycnospores (Wilk 3292, paratype). E. Ascospores. F. G. Cross-section of apothecium in regular (F) and polarized light (G). Bars: A = 2 mm; B, C = 100 µm; D, E = 10 µm; F, G = 200 µm.
