Burrowsia cataractae Fryday and I. Medeiros in Fryday, Medeiros, Siebert, Pope & Rajakaruna, S. Afr. J. Bot. 132: 473 (2020)
MycoBank number: MB 835879; Index Fungorum number: IF 835879; Facesoffungi number: FoF 14957;
Etymology — The specific epithet references the habitat of the only known collection, which is at the base of a waterfall: cataractae = of the waterfall (Latin: genitive, singular).
Thallus widespreading, grey—brown (greenish—brown when wet), rimose to cracked—areolate, margin obscurely lobed with a thin black prothallus, 0.3—0.4 mm thick; upper surface layer ±hyaline, cells with dilutely brown pigmented cap buried within an epinecral layer c. 10 μm deep; photobiont layer continuous, 20—70 μm deep; photobiont Trebouxia; cells chlorococcoid §orbicular 6—7 μm diam. to elongate 10—12 × 5—6μm; lower surface layer, c. 250 μm deep, brown pigmented, composed of §vertically aligned paraplecten chyma, cells 10 −15 × 5−6 μm. Apothecia frequent, lecideine, orbicular, occasionally flexuose when mature, dark brown to black, semi immersed to adnate with a broad base, becoming sessile, (0.4−)0.6−0.8 mm diam. Proper margin c. 0.1 mm broad, slightly raise and persistent, paler than the disk; in section cupular, initially brown but clearing and producing red, acicular crystals with K, in thin section with a brown, poorly defined cortex and ±hyaline medulla; composed of rows of radiating hyphae, 6—10 × 4—5 μm. Hymenium 110—130 μm, I+ blue; paraphyses §simple, rarely branched, sep- tate, becoming §moniliform above, 1.5—2.5 mm thick, widening to 3—4 μm at apex with a thin brown cap. Ascus cylindrical, c. 70 × (15—)20 μm, walls 2−3 μm thick; tholus IKI+ pale blue with a central, darker-staining tube-like structure narrowing towards the apex; ascospores 4—6(—8)/ascus, pigmented, submuri- form, non—halonate, (17—)23.61±4.37(—32) × (7—)11.74±5.66 (—15) μm, l/b ration (1.267—)2.05±0.39(—2.46), n = 23, ellipsoid, sometimes slightly curved. Hypothecium dark brown, c. 150μm thick, composed of ±randomly aligned hyphae. Conidiomata pycnidia, immersed in thallus with just dark ostiole visible, flask—shaped with pale brown wall; conidia long bacilliform, 10−12 × 1 μm Chemistry K+ red, C , Pd ; norstictic acid and two unidentified sub- stances by TLC. Both unidentified substances gave UVC++ pinkish-cream spots; a large one at Rf 7.0 and a smaller one at Rf 5.5 in solvent C.
Typus — South Africa, Mpumalanga, Ehlanzeni District, Thaba Chweu Municipality, Mashishing (Lydenburg), Buffelskloof Nature Reserve, Calodendrum Falls, 25° 17.839’S, 30° 30.656’E, 1500 m, damp closed forest, saxicolous boulders in splash zone of waterfall, 11 February 2016, A.M. Fryday (11 591), I. Medeiros, J. Burrows and A. Frisby (PRE—holo!) (Fig. 3).
GenBank accession numbers — nrLSU, MT611532; nrSSU, MT611986
Distribution and Ecology — The new species is known only from the type collection, which is at the base of a waterfall in a deep ravine (Fig. 4). The falls are on the Dwaalheuvel Quartzites and the new species grows on moist upper surfaces of flat, quartzite rocks around rock pools where it would have been permanently damp, if not peri- odically inundated. The vegetation of the ravine is classified as Eastern Dry Afrotemperate Forest (Lo€tter et al., 2014). This forest type receives mean annual precipitation of 1 084 mm and has a mean annual temperature of 16.7°C, but this is of little relevance to the highly humid microhabitat in which the new species occurred.
Notes — Separated from all other species in the Caliciaceae by its submuriform ascospores, ascus structure and DNA sequence data. Superficially similar to Rhizocarpon lavatum but with a different ascus structure, smaller pigmented submuriform ascospores and a thallus containing norstictic acid and two unidentified substances. The phylogenetic tree (Fig. 1) clearly places the new species in the Caliciaceae, albeit in an isolated position. It is included in a clade that also includes Buellia disciformis (Fr.) Mudd, which is the type species of Buellia, but with a very long branch length. The genus-level phylogeny and taxonomy of the buellioid Caliciaceae has not yet been settled, despite recent work on the family (Marbach, 2000; Prieto and Wedin, 2017). Species of Buellia typically have pigmented, 1-septate ascospores (rarely 3-sepate) and a different ascus structure (Bacidia or Biatora-type; Fig. 5). This type of ascus has a tholus (the area at the ascus apex between the inner and outer walls) that stains dark blue with an unstained central cone, either with (Biatora-type) or without (Bacidia-type) a darker staining wall (Rambold et al., 1994; Bungartz et al., 2007). Our new species would, therefore, be anoma- lous if placed in that genus. Submuriform ascospores are otherwise known in the Caliciaceae only in the genus Diplotomma Flot., which is well-separated from our new species in our phylogeny.
The gross morphology of the new species shows a remarkable similarity to Rhizocarpon lavatum (Ach.) Hazsl., which occurs in similar situations (damp, semi-inundated siliceous rocks) in the Northern Hemisphere (Fletcher et al., 2009) and has also been reported from New Zealand (Fryday, 2004), but not South Africa. However, the new species is readily separated from that species microscopically by the presence of pigmented ascospores and it also differs in its ascus structure and chemistry (Rhizocarpon-type and no substances in R. lavatum). We experienced some difficulty obtaining DNA sequence data from this species due to the frequent co-amplification of an endolichenic fungus in Chaetothyriales (Eurotiomycetes). Species of Chaetothyriales have recently been shown to be common endolichenic inhabitants of saxicolous lichens (Muggia et al., 2016) in addition to their previously documented role as extremophile rock-inhabiting fungi (Gueidan et al., 2008). The ITS and nrLSU sequences for the Trebouxia photobiont are available on GenBank as accessions MT622498 and MT611535, respectively.
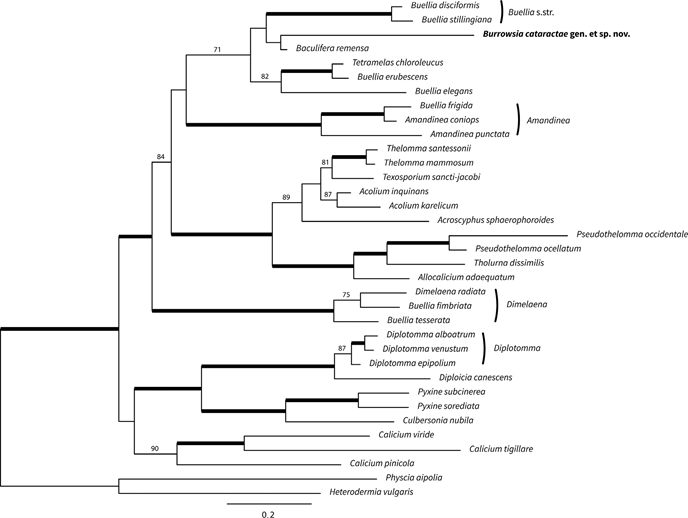
Fig. 1. Phylogeny of Caliciaceae based on maximum likelihood analysis of concatenated ITS, nrLSU, nrSSU, mtSSU, RPB1, RPB2, beta-tubulin and MCM7. Branches in bold have boot- strap support values ≥95; bootstrap values <70 are not displayed. Scale indicates substitutions per site. The new species is indicated with bold text.
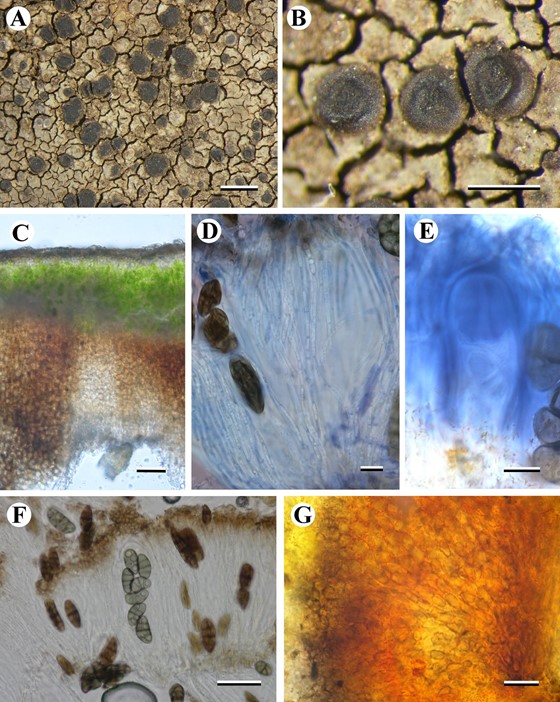
Fig. 3. Burrowsia cataractae (holotype). A: Thallus and apothecia, B: Immature apothecia, C: Thallus section, D: Paraphyses (in ink, pretreated with vinegar), E: Ascus (in IKI), F: Asco- spores, G: Exciple (in K). Scale bars: A & B = 1 mm; C, F & G = 20 mm; D & E = 10 mm.

Fig. 4. A: Calodendrum George on Buffelskloof Nature Reserve, B: Calodendrum Falls. Burrowsia cataractae was collected from rocks at the base of the falls (photographs by John Burrows).
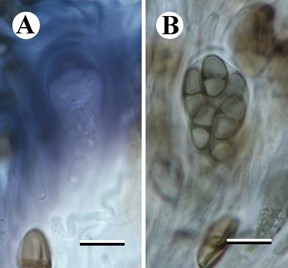
Fig. 5. Characters of typical buelluioid genera (B. gypsyensis Fryday). A, Ascus in IKI after pretreatment with K; B, ascospores in water. Scale bars = 10 mm.
